Mutant of endotoxin neutralizing peptide and application thereof
A peptide mutant, endotoxin technology, applied in the field of peptides, to achieve the effect of improving the activity of antagonizing LPS, increasing the number of positive charges and/or hydrophobicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
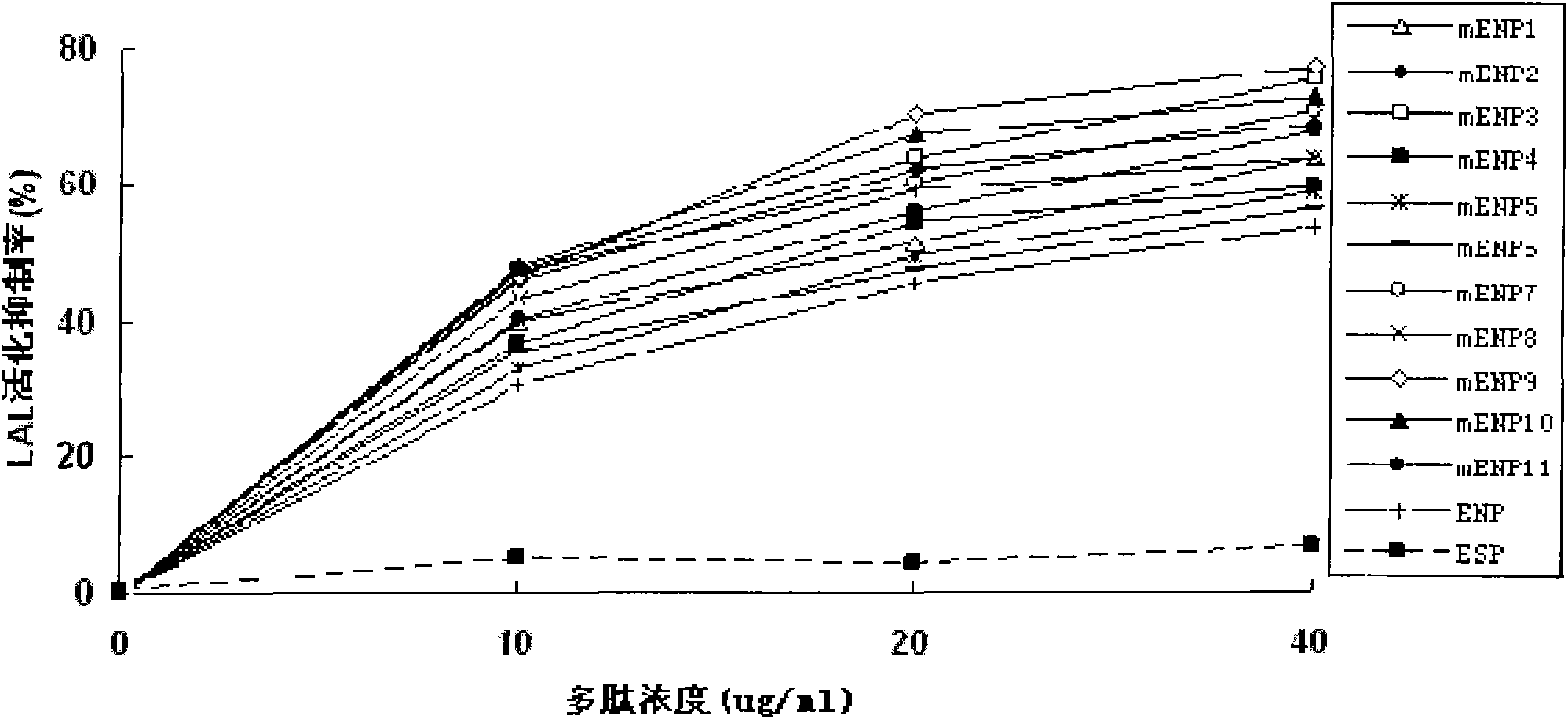
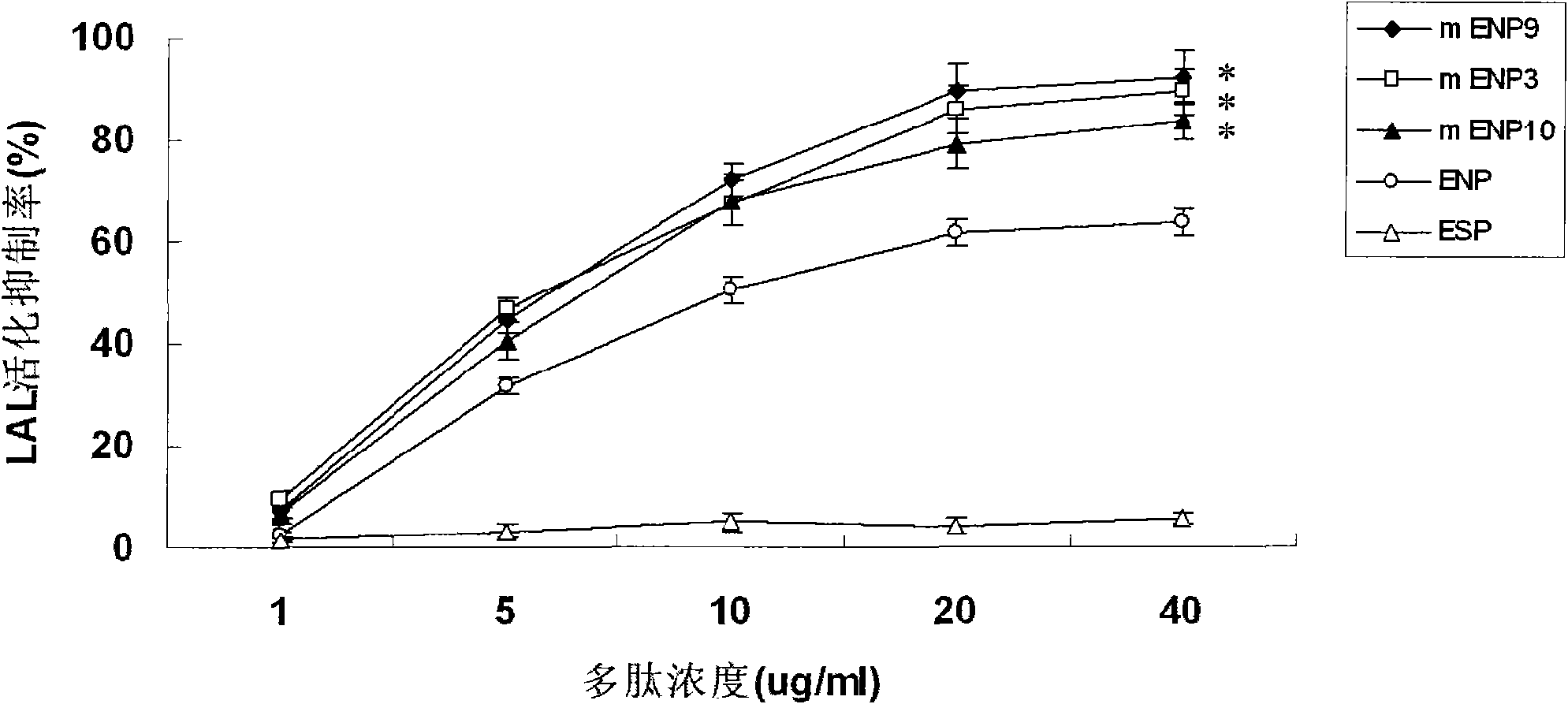
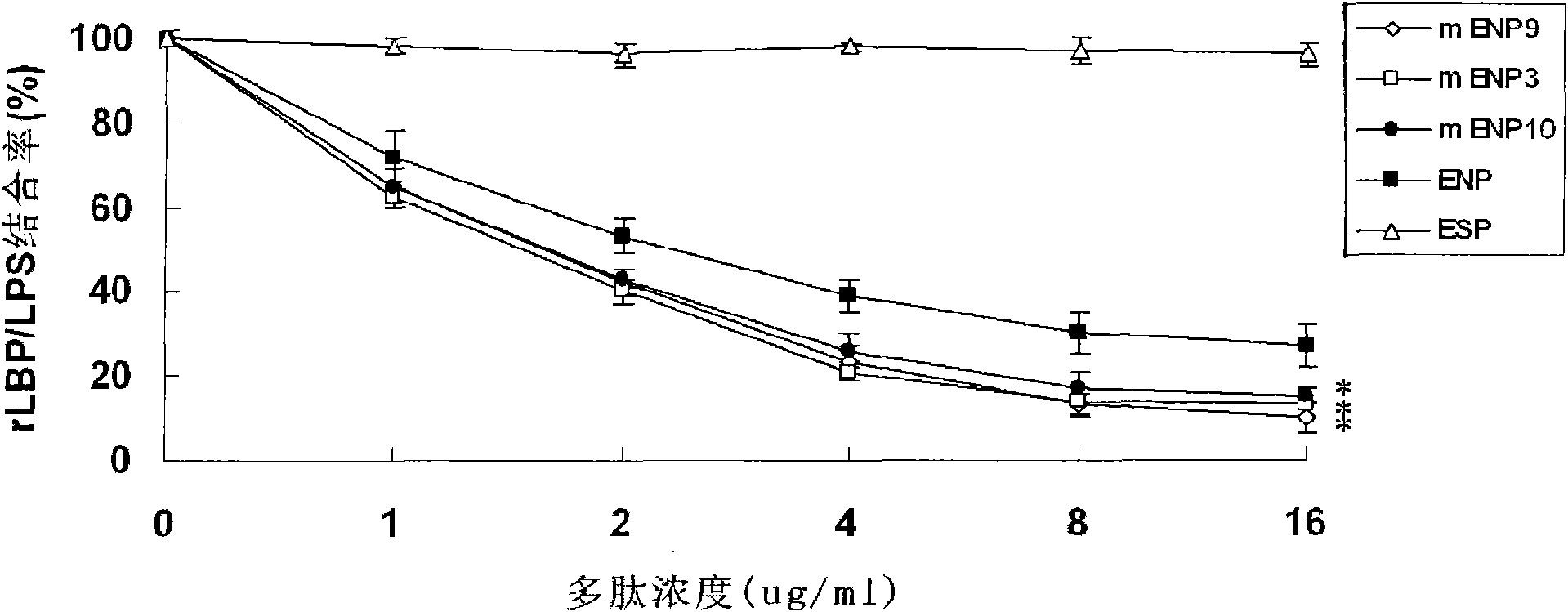
[0035] The preferred embodiments of the present invention will be described in detail below with reference to the accompanying drawings.
[0036] 1. Experimental materials
[0037] LPS (Escherichia coli, Escherichia coli, 0111:B4), myristate-phorbol-acetate (PMA) and polymyxin B (PMB) were purchased from Sigma (USA), CE TAL chromogenic substrate Limulus reagent The box (end-point chromogenic method) was purchased from Xiamen Limulus Reagent Experimental Factory Co., Ltd.; recombinant lipopolysaccharide-binding protein (rLBP) and anti-rLBP antibody were purchased from Cell Sciences (USA), cytotoxicity detection kit (Cell countingkit-8, CCK -8) Purchased from Dojindo laboratories (JAPAN); BCA protein detection kit was purchased from Pierce Biotechnology (USA), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) detection kits were purchased from Nanjing Jiancheng Biology Institute of Engineering; human tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6) enz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com