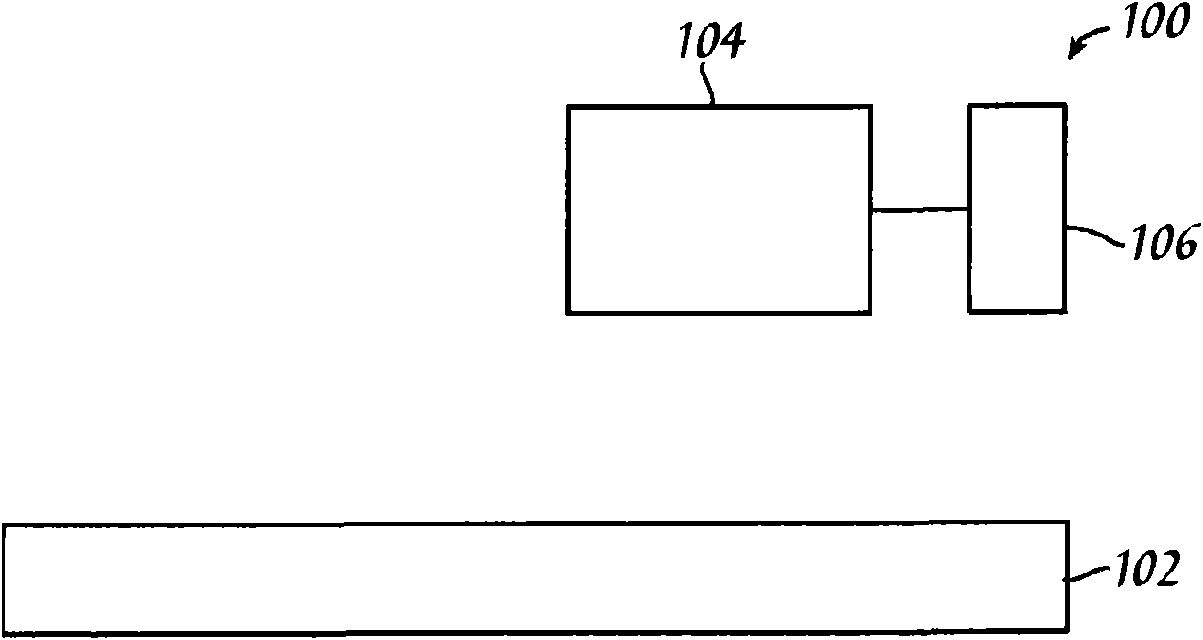
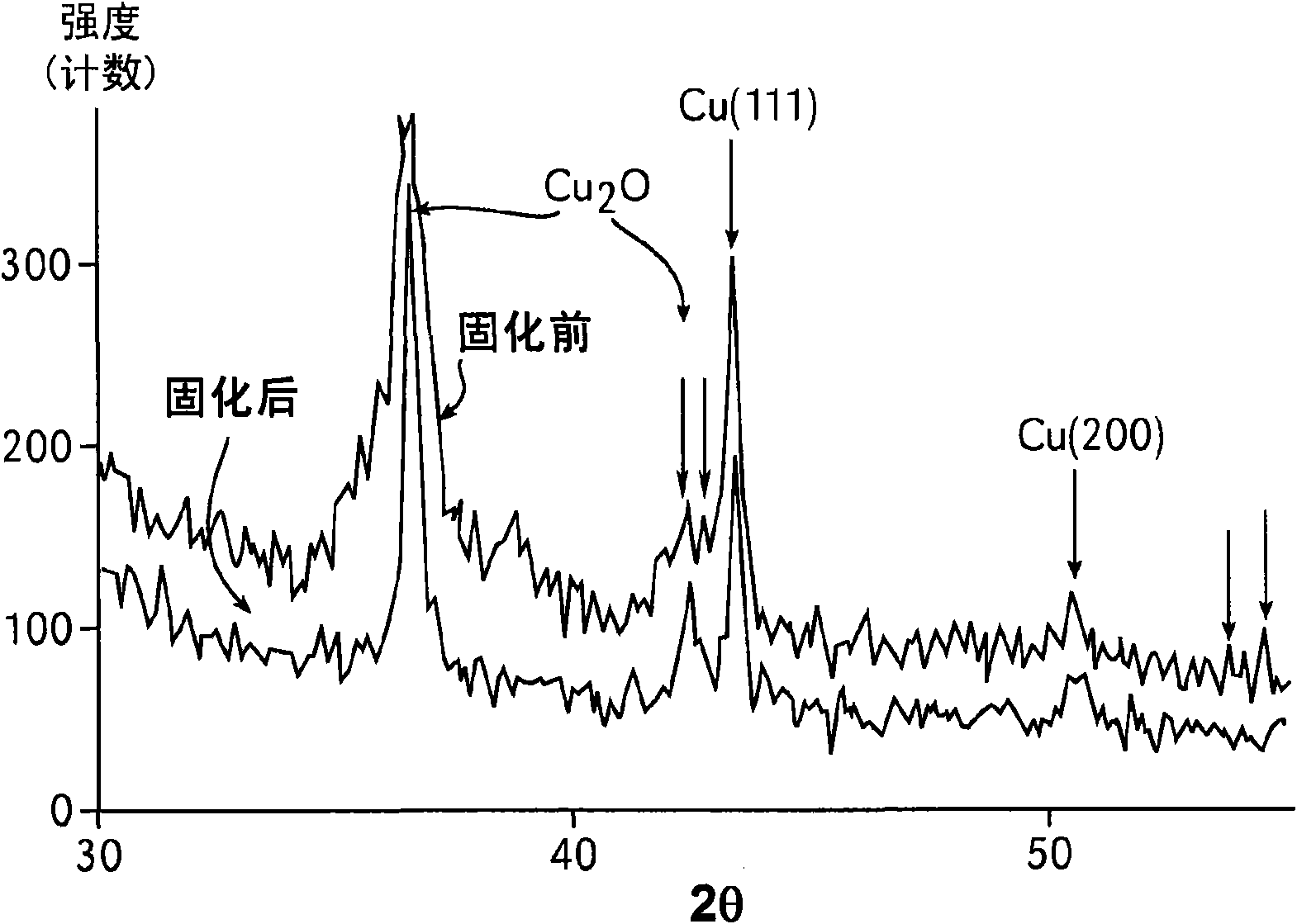
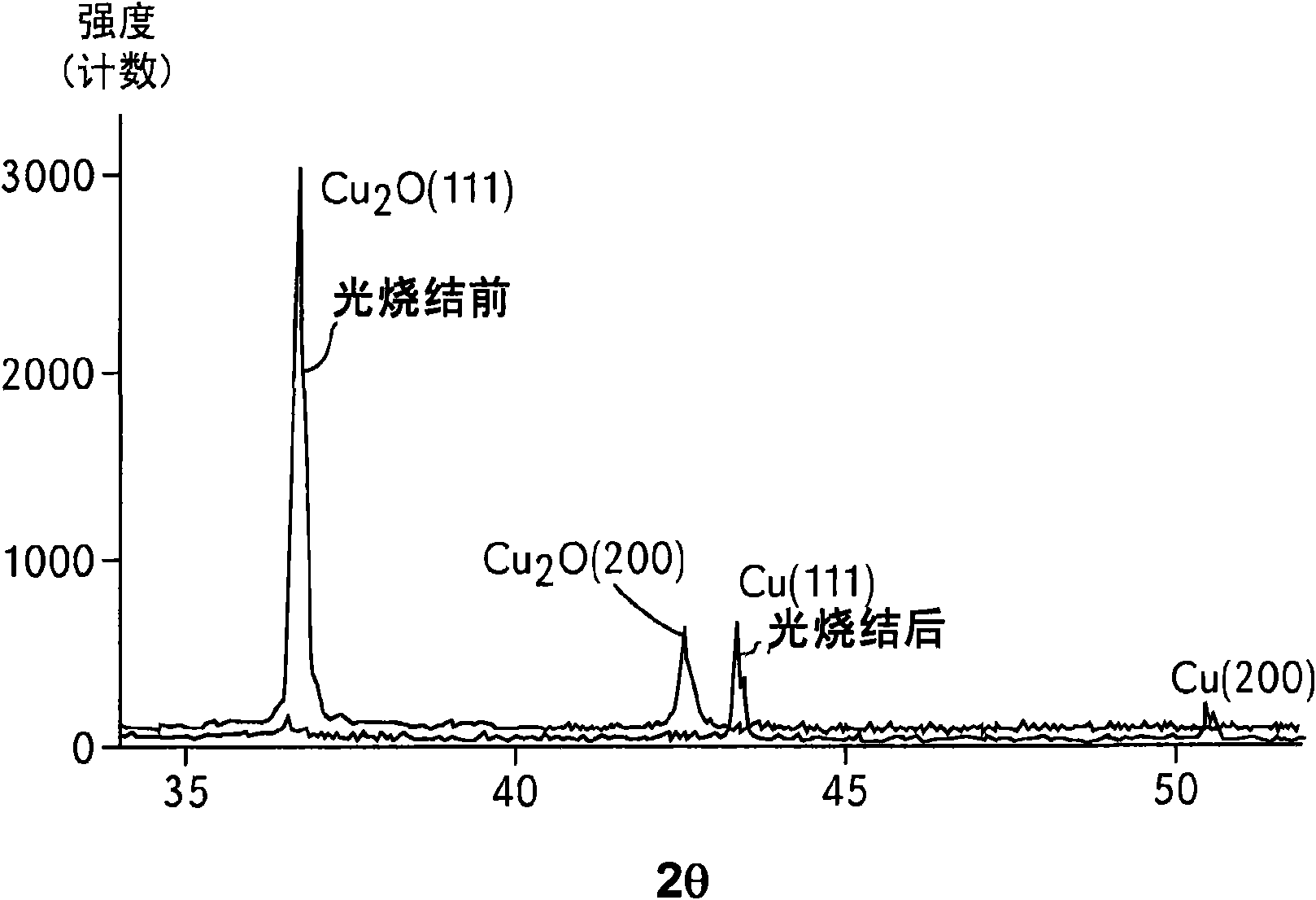
Metallic ink
A nanoparticle ink, a part of the technology, used in metal processing equipment, conductors, transportation and packaging, which can solve problems such as cost barriers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0141] Embodiment 1 is used for the nonionic polymer dispersant of copper printing ink
[0142] The "excluded" volume represents the space "occupied" by the flexible alkyl (or ethoxy) chain, which cannot be occupied by a second copper nanoparticle. The larger the "excluded" volume, the better the dispersant is at separating the copper nanoparticles. A second aspect of this "excluded" volume is that, at larger values, lower concentrations of the compound are effective in providing high coverage of the nanoparticles, and thus are more effective dispersants.
[0143] Polymers that can be successfully used to formulate inks and then cure by photonic curing to obtain good conductors are: Triton X-100, Triton X-15, Triton X-45, Triton QX-15, linear alkyl ether (colar CapMA259 , colar Cap MA1610), quaternized alkyl imidazolines (Cola Solv IES and Cola SolvTES), polyvinylpyrrolidone (PVP), polyvinyl alcohol, polyethylene glycol and polysiloxanes. When preparing copper ink with coppe...
Embodiment 2
[0146] Embodiment 2 is used for the ionic polymer dispersion agent of copper printing ink
[0147] Copper inks were formulated with a compatible carrier using acidic group-containing copolymers as dispersants. Copolymers containing ionic groups, such as Disperbyk 180, Disperbyk 111, styrene-maleic anhydride copolymer (SMA 1440, flake), PT, HCS-P, HCS-N, etc., have ionic properties and thus obtain electrostatic dispersion . When formulating copper ink with copper nanoparticles, a compatible carrier or its combination can be used at the same time, such as 2-butoxyethyl acetate, propylene glycol monomethyl ether acetate, diethylene glycol monoethyl ether acetate , Ethylene glycol butyl ether, cyclohexanone, cyclohexanol, 2-ethoxyethyl acetate, ethylene glycol diacetate, terpineol, isobutanol, etc. Copper ink jet printing on flexible substrates. Then, at low temperatures below 150°C, a pre-cure method is used in air to remove as much vehicle and dispersion as possible. Finally...
Embodiment 3
[0149] Embodiment 3 is formulated with nonionic dispersant and ionic dispersant
[0150] In order to obtain a better dispersion effect, both non-ionic and ionic dispersants are used to prepare copper inks. When preparing copper ink, non-ionic dispersants can be used at the same time, such as Triton X-100, TritonX-15, Triton X-45, Triton QX-15, linear alkyl ethers (colar Cap MA259, colar Cap MA1610), quaternary ammonium alkylated imidazolines (Cola Solv IES and Cola Solv TES), polyvinylpyrrolidone (PVP); copolymers containing ionic groups, such as Disperbyk 180, Disperbyk 111, etc.; and styrene-maleic anhydride copolymers (SMA 1440, sheet), PT, HCS-P, HCS-N, etc. Copper nanoparticles passivated with copper oxide were used in the formulation. When preparing copper ink with copper nanoparticles, compatible carriers can be selected, such as 2-butoxyethyl acetate, propylene glycol monomethyl ether acetate, diethylene glycol monoethyl ether acetate, 2-ethyl acetate Oxyethyl ester...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com