Supported catalyst for hydrogenation/dehydrogenation reaction, method for production of the catalyst, and hydrogen storage/supply method using the catalyst
A catalyst, dehydrogenation technology, applied in catalyst activation/preparation, metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, etc., can solve the time lag, lack of reformer responsiveness, practicality Low-level problems, to achieve the effect of improving the response speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0063] Examples of the present invention will be described below, but the present invention is not limited to these Examples.
[0064] experiment method
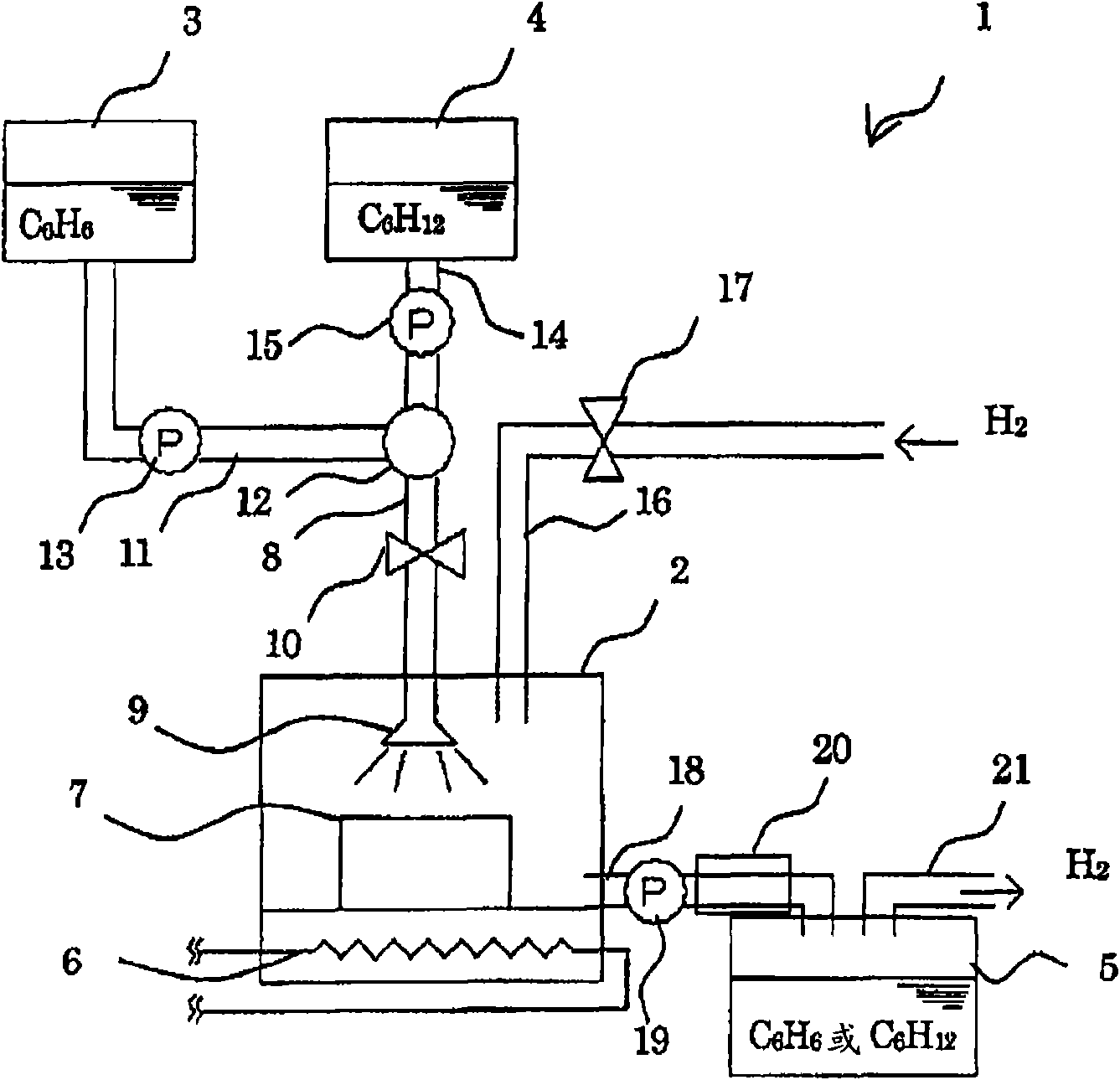
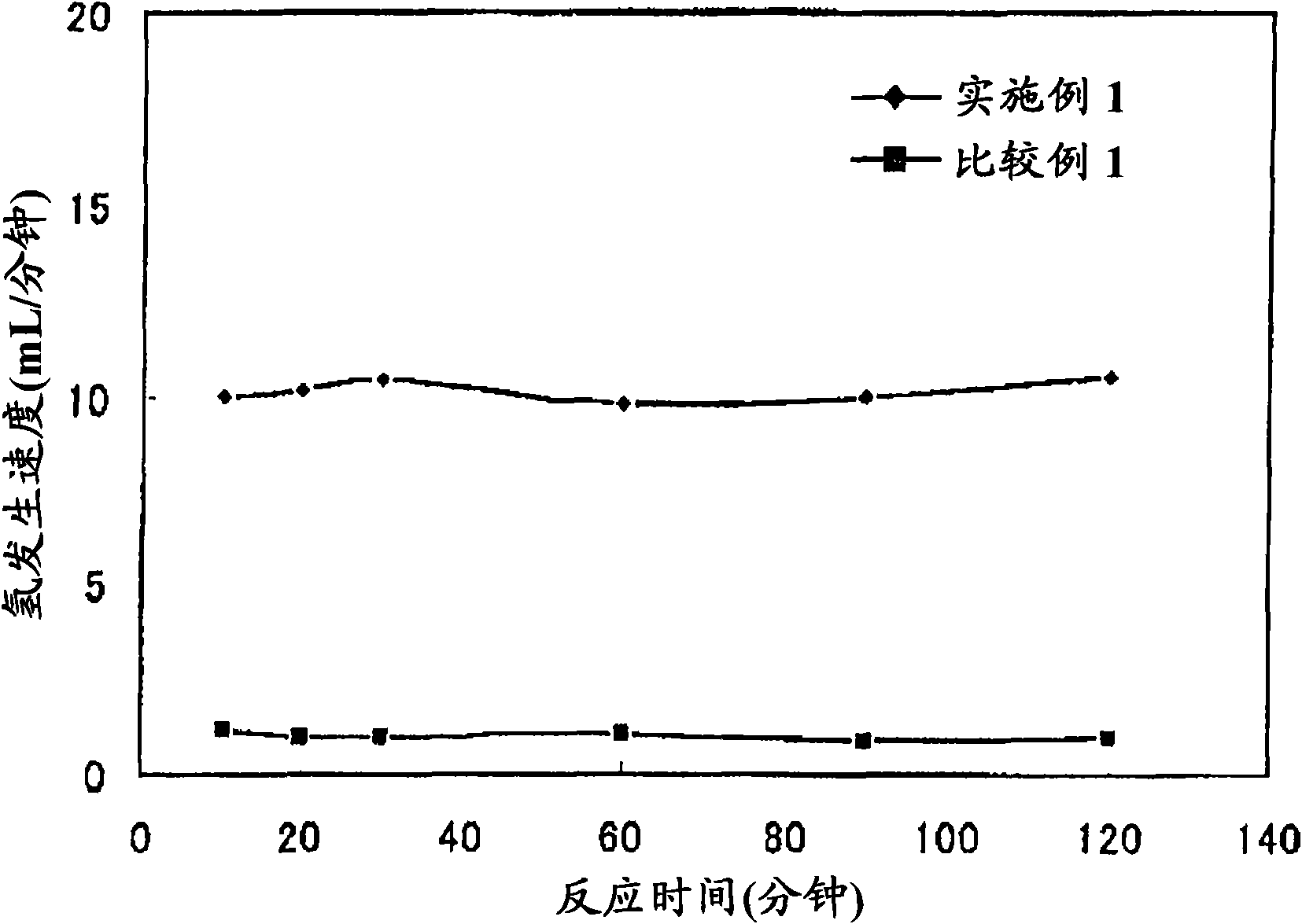
[0065] exist figure 1 In the shown hydrogenation and dehydrogenation reaction apparatus, the dehydrogenation reaction of cyclohexane or methylcyclohexane was carried out using the samples of each of Examples and Comparative Examples described later. The reaction conditions are set as follows: temperature 320°C, reaction pressure 0.1MPaG, catalyst layer volume 4.3ml, raw material addition rate 0.15ml / min, liquid air velocity 2.0h -1 , The amount of hydrogen added is 10ml / min (hydrogen / oil ratio is 0.3mol / 1mol). The rate of hydrogen generation and the conversion rate of cyclohexane were determined by gas chromatographic analysis. When the dehydrogenation reaction of cyclohexane is carried out, the hydrogen generation rate, cyclohexane conversion rate and benzene selectivity are shown in Table 1. When carrying out the dehyd...
Embodiment 1
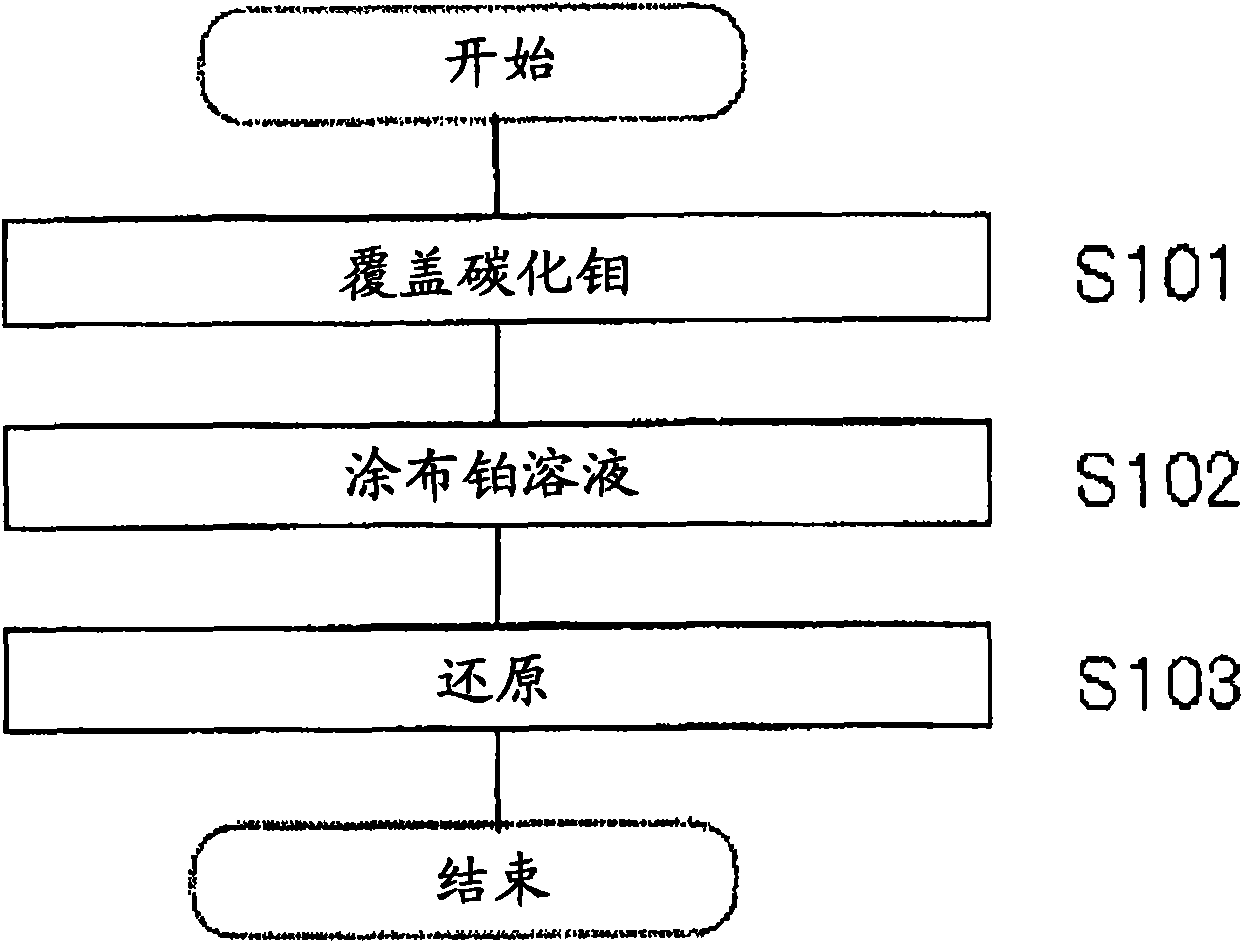
[0072] Chloroplatinic acid H containing 18 mg platinum 2 PtCl 6 The aqueous solution was sprayed onto the molybdenum carbide, followed by firing at 350° C. for 3 hours in a nitrogen atmosphere. Then, in a 10% hydrogen atmosphere diluted with nitrogen, the temperature was raised from room temperature to 350 ° C in stages to produce molybdenum carbide (Pt / Mo) loaded with 0.5% platinum. 2 C) Catalyst.
Embodiment 2-5
[0074] Activated carbon, mesoporous silicate (FSM-16), silica and alumina were used as supports respectively, and a solution containing ammonium molybdate was coated on the support, and then heated at 350° C. under an air atmosphere. Activated carbon loaded with molybdenum oxide, mesoporous silicate, silicon dioxide and aluminum oxide are separated at 350-500 ° C under the mixed gas atmosphere of methane (or butane): hydrogen = 1:10 according to the volume ratio. The heating reaction was carried out for 5 hours in stages, thereby converting molybdenum oxide into molybdenum carbide.
[0075] Each supported catalyst covered with molybdenum carbide was then impregnated in chloroplatinic acid H containing 18 mg platinum. 2 PtCl 6 solution and then dried. Then hydrogen reduction was carried out step by step at 100-350° C. for 3 hours under a hydrogen atmosphere to prepare various supported catalysts (Example 2-5).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com