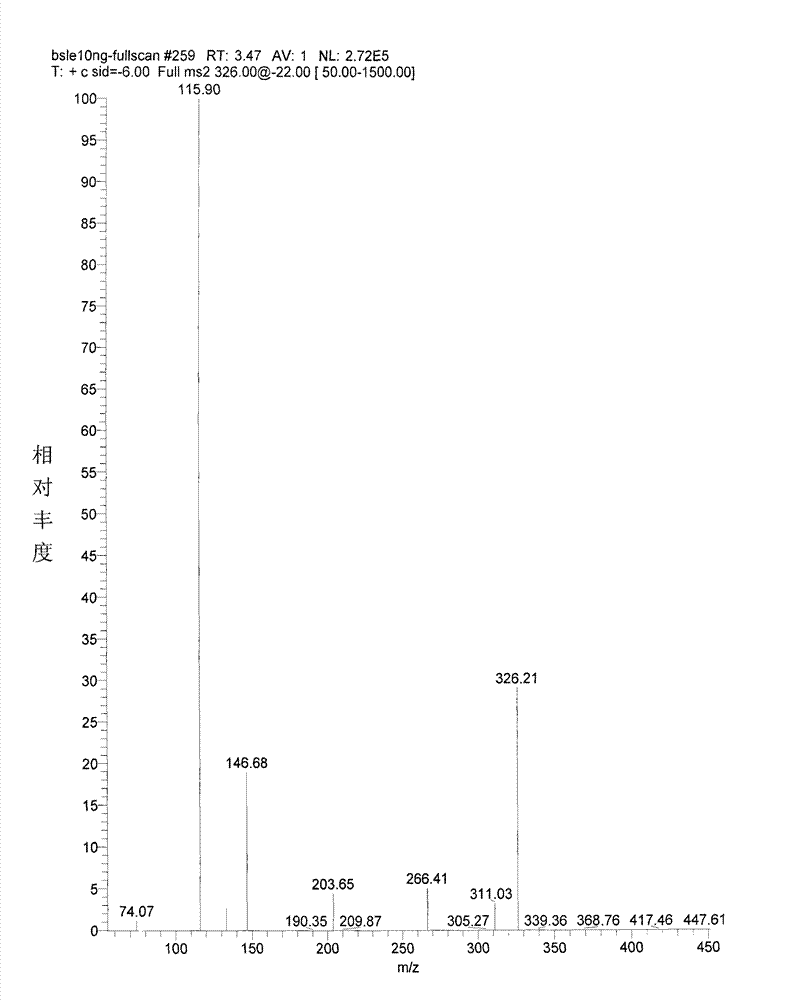
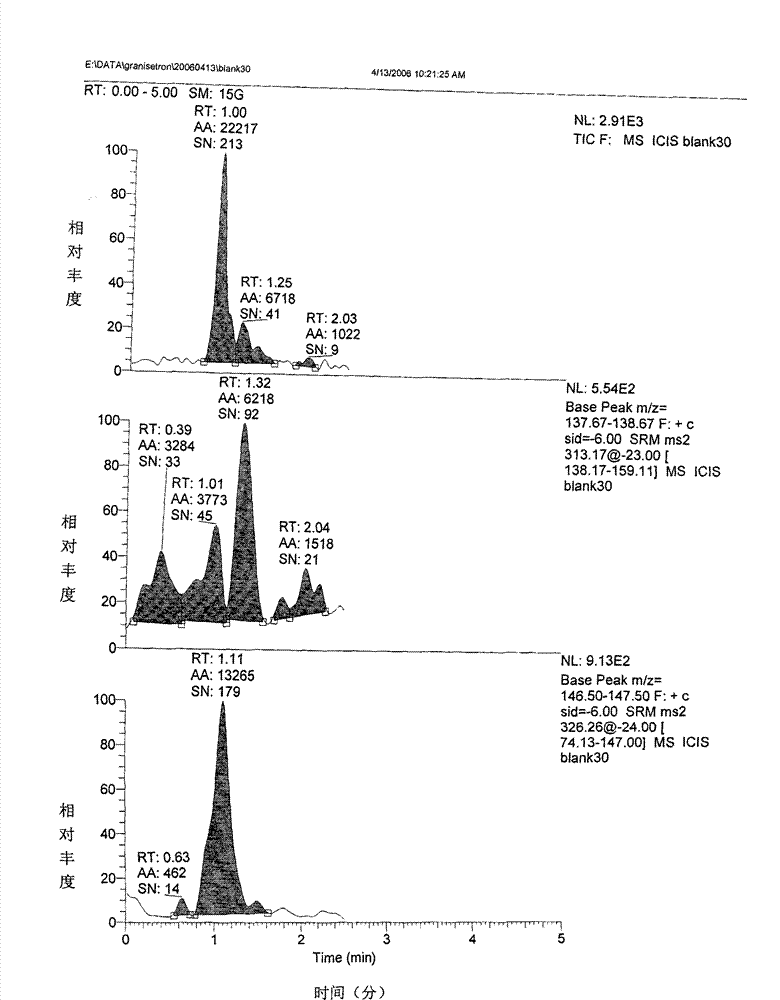
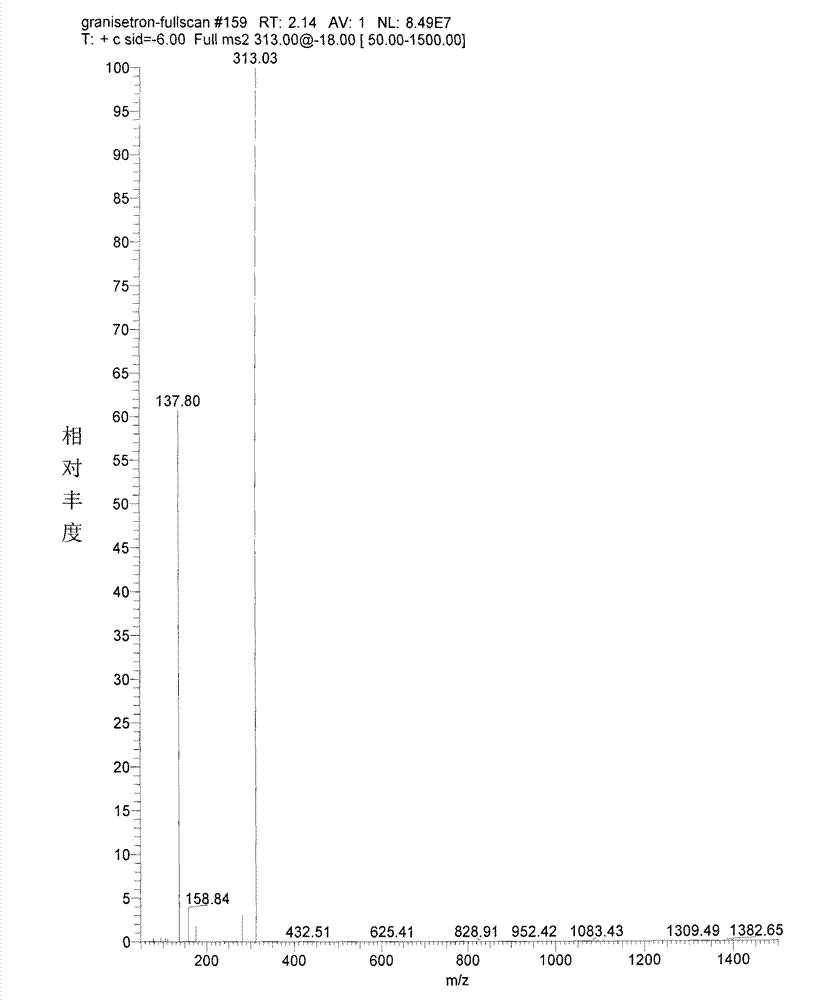Granisetron hydrochloride nasal spray and preparation method thereof
A technology of granisetron hydrochloride nasal cavity and granisetron hydrochloride, applied in aerosol delivery, digestive system, drug combination, etc., can solve the problems of low proteolytic enzyme activity, low bioavailability, inconvenient use, etc., and achieve improvement Compliance, ease of administration, and improved absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] The raw materials are: 10 parts of granisetron hydrochloride, 10 parts of ethylparaben, 10 parts of polyoxyethylene-9-lauryl ether, 5 parts of thiolauryl alcohol, 10 parts of 1,2-propylene glycol, 10 parts of glycerin, purified water Right amount, right amount of fragrance.
[0069] The preparation method is as follows:
[0070] (1) Take 10 parts of granisetron hydrochloride and 10 parts of ethylparaben, pulverize them, and pass through an 80-mesh sieve; place them in a volumetric flask, add an appropriate amount of purified water, and shake fully;
[0071] (2) Add 10 parts of polyoxyethylene-9-lauryl ether, 5 parts of thiolauryl alcohol, 10 parts of 1,2-propylene glycol, 10 parts of glycerin, and an appropriate amount of essence to the solution prepared in the first step;
[0072] (3) Add purified water to the scale, shake to make the solution mix evenly;
[0073] (4) Filling, sealing and packaging to obtain granisetron hydrochloride nasal cavity spray.
Embodiment 2
[0075] The raw materials are: 10 parts of granisetron hydrochloride, 20 parts of ethylparaben, 20 parts of polyoxyethylene-9-lauryl ether, 5 parts of thiolauryl alcohol, 10 parts of 1,2-propylene glycol, 10 parts of glycerin, purified water Right amount, right amount of fragrance.
[0076] The preparation method is as follows:
[0077] (1) Take 10 parts of granisetron hydrochloride and 20 parts of ethylparaben, pulverize them, and pass through an 80-mesh sieve; place them in a volumetric flask, add an appropriate amount of purified water, and shake fully;
[0078] (2) Add 20 parts of polyoxyethylene-9-lauryl ether, 5 parts of thiolauryl alcohol, 10 parts of 1,2-propylene glycol, 10 parts of glycerin, and an appropriate amount of essence to the solution prepared in the first step;
[0079] (3) Add purified water to the scale, shake to make the solution mix evenly;
[0080] (4) Filling, sealing and packaging to obtain granisetron hydrochloride nasal cavity spray.
Embodiment 3
[0082] The raw materials are: 12 parts of granisetron hydrochloride, 15 parts of ethylparaben, 18 parts of polyoxyethylene-9-lauryl ether, 8 parts of thiolauryl alcohol, 13 parts of 1,2-propylene glycol, 15 parts of glycerin, purified water Right amount, right amount of fragrance.
[0083] The preparation method is as follows:
[0084] (1) Take 12 parts of granisetron hydrochloride and 15 parts of ethylparaben, pulverize them, and pass through an 80-mesh sieve; place them in a volumetric flask, add an appropriate amount of purified water, and shake fully;
[0085] (2) Add 18 parts of polyoxyethylene-9-lauryl ether, 8 parts of thiolauryl alcohol, 13 parts of 1,2-propylene glycol, 15 parts of glycerin, and an appropriate amount of essence to the solution prepared in the first step;
[0086] (3) Add purified water to the scale, shake to make the solution mix evenly;
[0087] (4) Filling, sealing and packaging to obtain granisetron hydrochloride nasal cavity spray.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com