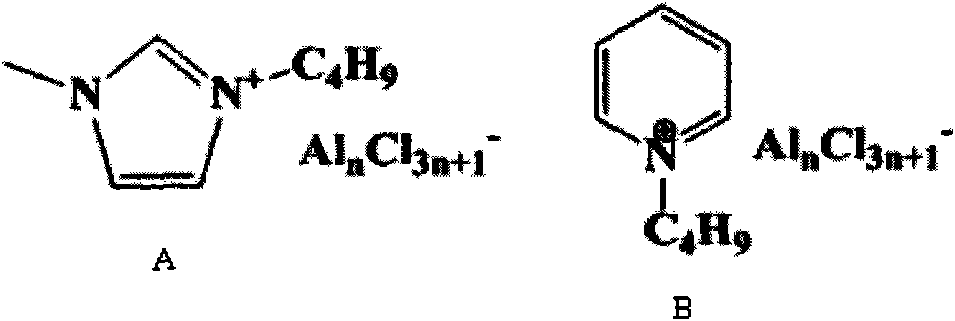Method for preparing acetyl salicylamide
A technology of acetylsalicylic amide and salicylic amide, applied in the field of preparing acetylsalicylic amide, can solve problems such as non-reusable, unpleasant taste, pollute the environment, etc., and achieve the effect of saving production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation of ionic liquid (structural formula sees specification attached figure 1 )
[0028] 1-Butyl-3-methylimidazolium chloroaluminate [BMIM]Cl-nAlCl 3 (n=1.5-2.5) Preparation method of ionic liquid: under nitrogen protection, in a 500mL three-necked flask with a reflux condenser, 1.50mol (123g) of 1-methylimidazole, 1.7mol (158g) of The alkane was stirred and warmed up to 80°C, and the reaction was refluxed for 48h. Then cool at 0°C for 12h to crystallize, pour off the remaining unreacted liquid, then add 20mL of ethyl acetate to wash, the crude product is rotary evaporated at 80°C to remove residual ethyl acetate, and then dried in vacuum at 70°C for 24h. The yield of the product [BMIM]Cl is about 90%, and it is stored in a dry and inert atmosphere for future use.
[0029] The glove box was filled with nitrogen, and different amounts of anhydrous AlCl were slowly added in batches to the quantitative above-mentioned intermediate [BMIM]Cl at room temperature...
Embodiment 2
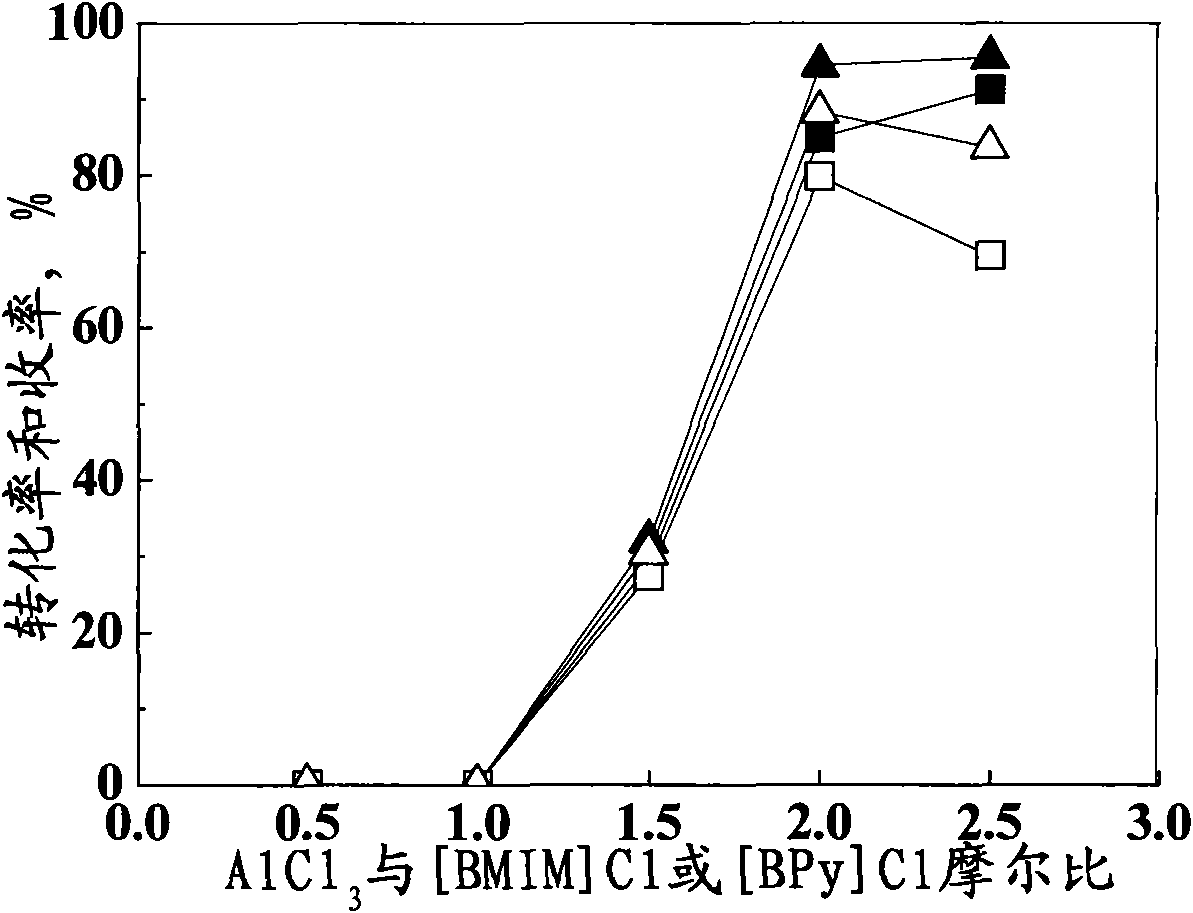
[0039] Same as Example 1 but keep the ionic liquid composition as n=2.0, only change the ionic liquid consumption to be 20mmol, 60mmol, 80mmol respectively, the results are shown in image 3 .
[0040] Such as image 3 Shown, when ionic liquid consumption is 20mmol (being promptly with salicylamide mol ratio 1: 1), due to reason such as stirring difficulty, cause the conversion rate of salicylamide and the yield of acetylsalicylamide to be all low; The increase of ionic liquid consumption, the conversion rate of salicylamide constantly increases, but after surpassing 40mmol, it is not obvious, and because the increase of side reaction, the yield of acetylsalicylamide declines instead, therefore, optimal ionic liquid consumption is 40mmol ( That is, the molar ratio with salicylamide is 2:1).
Embodiment 3
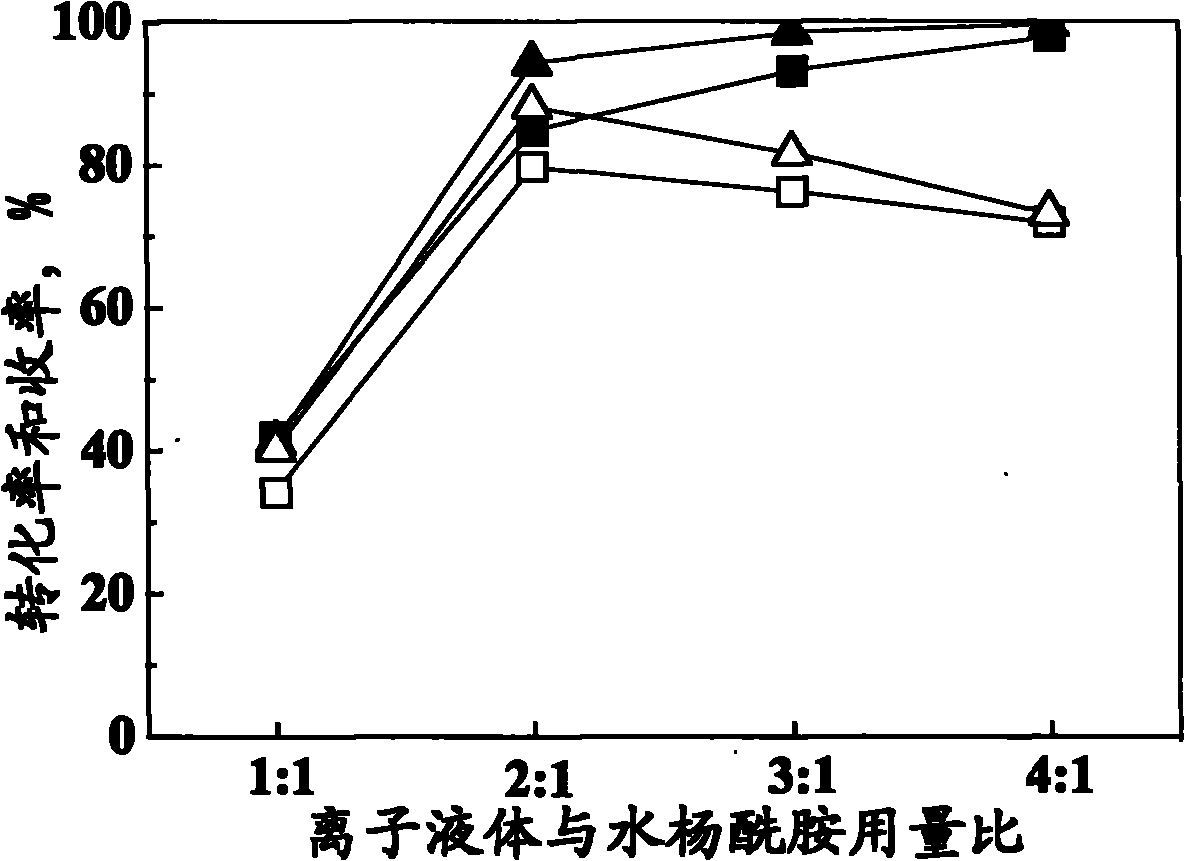
[0042] Same as Example 1 but keep the ionic liquid composition as n=2.0, only change the amount of acetyl chloride to be 20mmol, 60mmol, 80mmol respectively, the results are shown in Figure 4 .
[0043] Such as Figure 4 As shown, when the amount of acetyl chloride was 20mmol (i.e. 1:1 with salicylamide molar ratio), due to volatilization, partial hydrolysis and other reasons, the conversion rate of salicylamide and the yield of acetylsalicylamide were all low; Increase the amount of acetyl chloride to 40mmol (that is, the molar ratio of salicylamide to 2: 1), the conversion rate of salicylamide and the yield of acetyl salicylamide all increase, but not obviously, continue to increase the amount of acetyl chloride, salicylamide On the contrary, the conversion rate and the yield of acetylsalicylamide decrease slightly, therefore, the optimal amount of acetyl chloride is 20-40mmol (that is, the molar ratio to salicylamide is 1:1-2:1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com