Method for producing propylene from methanol or dimethyl ether
A dimethyl ether and propylene technology, applied in ethylene production, bulk chemical production, chemical instruments and methods, etc., can solve the problems of unstable operation, fluctuating reaction conditions, catalyst deactivation, etc., to achieve stable operation and maintain stable activity performance, solve the effect of frequent switching and regeneration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
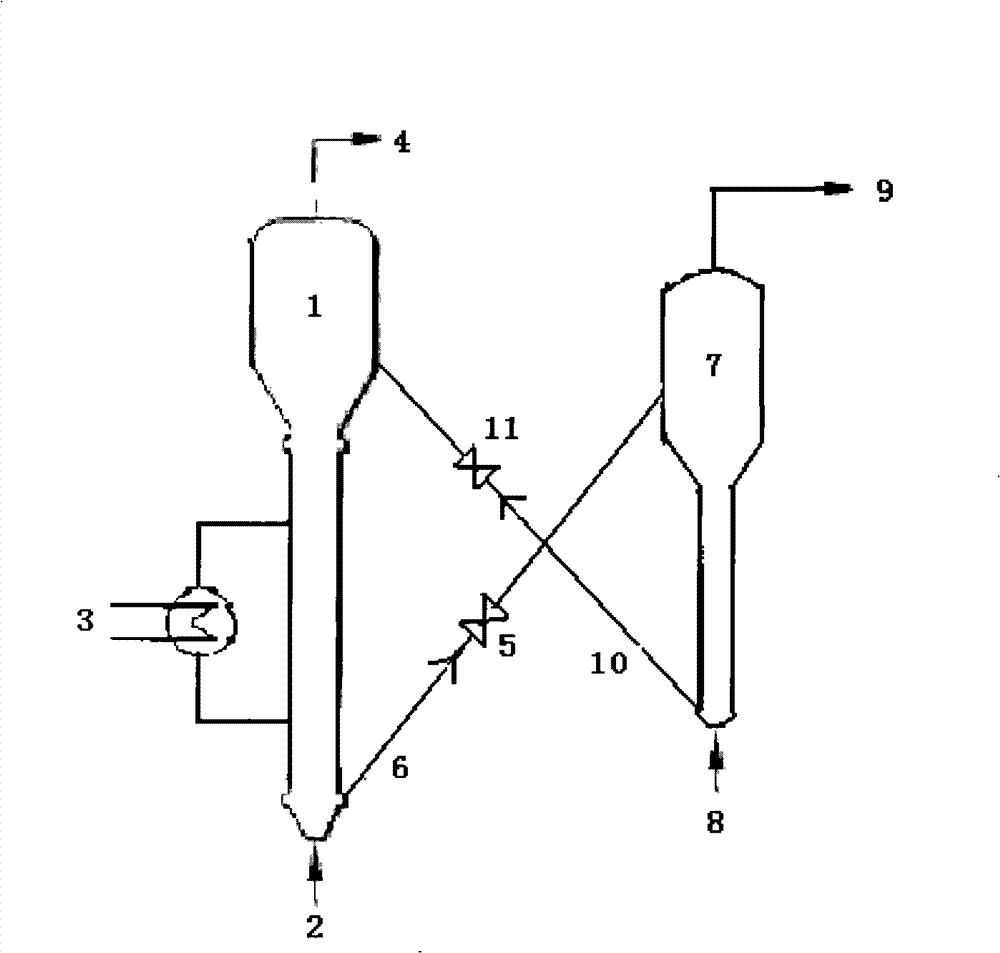
[0027] according to figure 1 In the process flow shown, the raw material methanol enters the reactor 1 from the inlet 2, and the heat generated by the reaction is taken out through the heat exchanger 3, and the partially deactivated ZSM-5 molecular sieve (SiO 2 / Al 2 o 3 The molar ratio is 50), and the catalyst is controlled by the catalyst control slide valve 5, and enters the regenerator 6 from the catalyst standby inclined pipe. In the regenerator 6, the deactivated catalyst is regenerated and activated by the air coming in from the regeneration air inlet 8, and the catalyst recovers its activity. The exhaust gas generated by regeneration is discharged from the exhaust gas outlet 8 of the regenerator. The regenerated catalyst is controlled by the catalyst control slide valve 9, and returned to the reactor 1 for recycling through the regeneration inclined pipe. The pressure in the reactor is 0.02MPa, the reaction temperature is 400°C, and the regeneration temperature is 5...
Embodiment 2
[0029] according to figure 1 In the process flow shown, the raw material dimethyl ether enters the reactor 1 from the inlet 2, and the heat generated by the reaction is taken out through the heat exchanger 3, and the partially deactivated ZSM-5 molecular sieve (SiO 2 / Al 2 o 3 The molar ratio is 150) the catalyst is controlled by the catalyst control slide valve 5, and enters the regenerator 6 from the catalyst standby inclined tube. In the regenerator 6, the deactivated catalyst is regenerated and activated by the air coming in from the regeneration air inlet 8, and the catalyst recovers its activity. The exhaust gas generated by regeneration is discharged from the exhaust gas outlet 8 of the regenerator. The regenerated catalyst is controlled by the catalyst control slide valve 9, and returned to the reactor 1 for recycling through the regeneration inclined pipe. The pressure in the reactor is 0.1MPa, the reaction temperature is 500°C, and the regeneration temperature is ...
Embodiment 3
[0031] according to figure 1 In the process flow shown, the mixture of raw material methanol and water (water / methanol=0.5) enters the reactor 1 from the inlet 2, and the heat generated by the reaction is taken out through the heat exchanger 3, and the partially deactivated ZSM-5 molecular sieve (SiO 2 / Al 2 o 3The molar ratio is 100) the catalyst is controlled by the catalyst control slide valve 5, and enters the regenerator 6 from the catalyst standby inclined tube. In the regenerator 6, the deactivated catalyst is regenerated and activated by the air coming in from the regeneration air inlet 8, and the catalyst recovers its activity. The exhaust gas generated by regeneration is discharged from the exhaust gas outlet 8 of the regenerator. The regenerated catalyst is controlled by the catalyst control slide valve 9, and returned to the reactor 1 for recycling through the regeneration inclined pipe. The pressure in the reactor is 0.05MPa, the reaction temperature is 450°C, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com