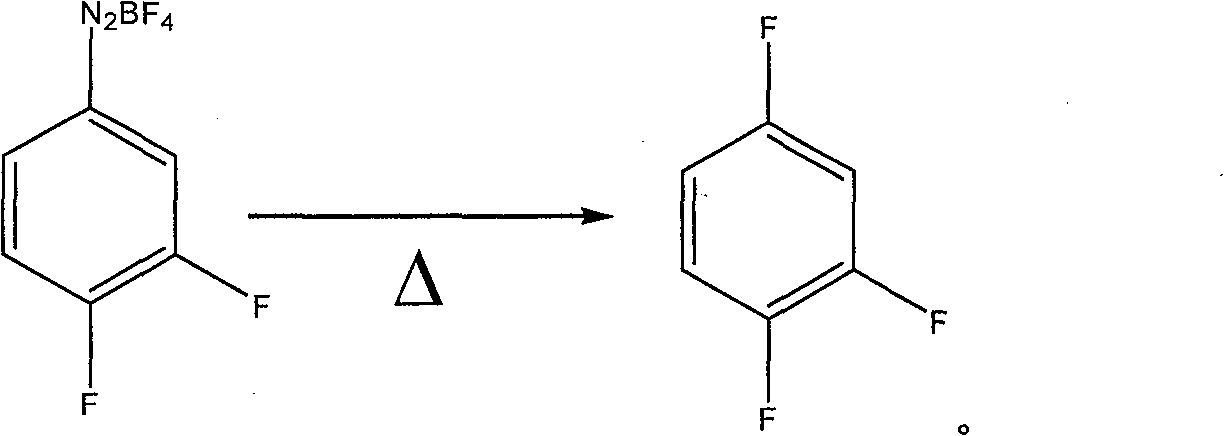
Preparation method of 1,2,4-trifluoro-benzene
A technology of trifluorobenzene and difluoroaniline, which is applied in the preparation of halogenated hydrocarbons, chemical instruments and methods, organic chemistry, etc., can solve the problems of difficult industrial production of hexafluorobenzene, harsh reaction conditions, and difficult industrial production , to achieve the effect of being suitable for large-scale industrial production, mild reaction conditions, and saving raw material costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
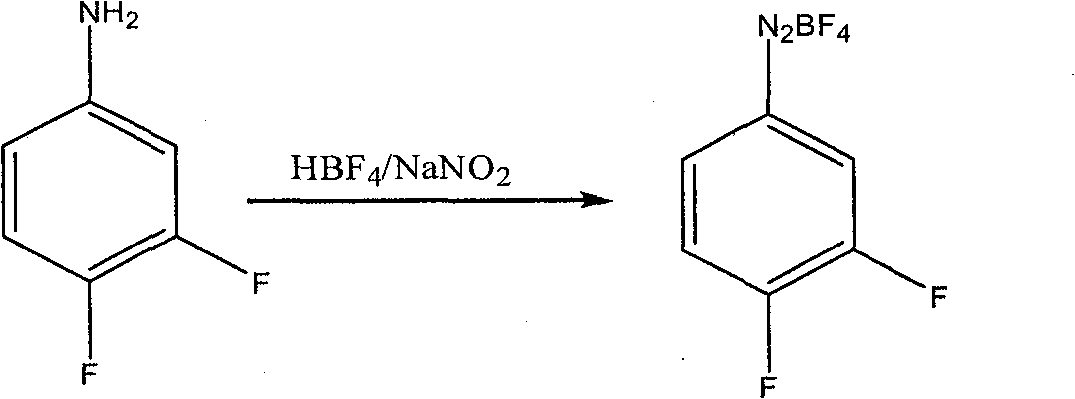
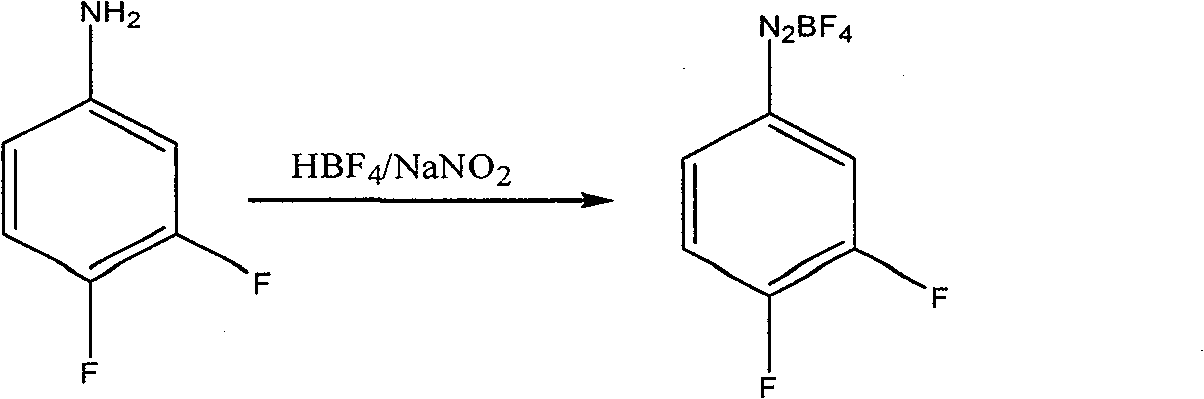
[0020] Example 1: Preparation of diazonium fluoroborate
[0021] Add 30 grams of 3.4-difluoroaniline into a 100ml four-necked reaction flask, add dropwise 153 grams of fluoboric acid with a concentration of 40% by mass at 25°C to 30°C, and directly quench with ice-salt water to - 15 ℃ ~ 0 ℃, and maintain this temperature slowly dropwise sodium nitrite aqueous solution (17.7 grams of sodium nitrite and 25 grams of water), keep warm for 1 to 2 hours after the dropwise addition, suction filtration, filter cake with frozen 40% Fluoroboric acid aqueous solution 10ml washes, and then with frozen ethanol 20ml, 20ml washes twice, filter cake carries out vacuum distillation to remove solvent, obtains 40 grams of fluoboric acid diazonium salts.
Embodiment 2
[0022] Example 2: Preparation of diazonium fluoroborate
[0023] All the steps in this example are the same as in Example 1, the difference is that 143 grams of fluoboric acid with a concentration of 40% by mass is added dropwise at 0°C to 20°C, and after the dropwise addition, it is directly quenched to -40°C with ice salt water. ℃~5℃, 42 grams of diazonium fluoroborate were obtained.
Embodiment 3
[0024] Example 3: Preparation of diazonium fluoroborate
[0025] All the steps in this example are the same as in Example 1, the difference is that 153 grams of fluoboric acid with a concentration of 40% by mass is added dropwise at 15°C to 35°C, and after the dropwise addition, it is directly quenched to -25°C with ice salt water. ℃~0℃, maintain this temperature and slowly add sodium nitrite aqueous solution (16.8 grams of sodium nitrite and 25 grams of water) dropwise to obtain 42.5 grams of diazonium fluoroborate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com