Method for screening new internal reference molecules suitable for cervical tissue micro RNA real-time fluorescence quantitative PCR research
A technology for real-time fluorescence quantification and screening methods, applied in fluorescence/phosphorescence, biochemical equipment and methods, material excitation analysis, etc., it can solve the problem of unexplored constancy of expression and the applicability of internal reference, and improve accuracy and reliability. The effect of stability, good calibration and normalization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027] Below in conjunction with accompanying drawing, the present invention is further explained and illustrated:
[0028] Such as Figure 1-8 Shown:
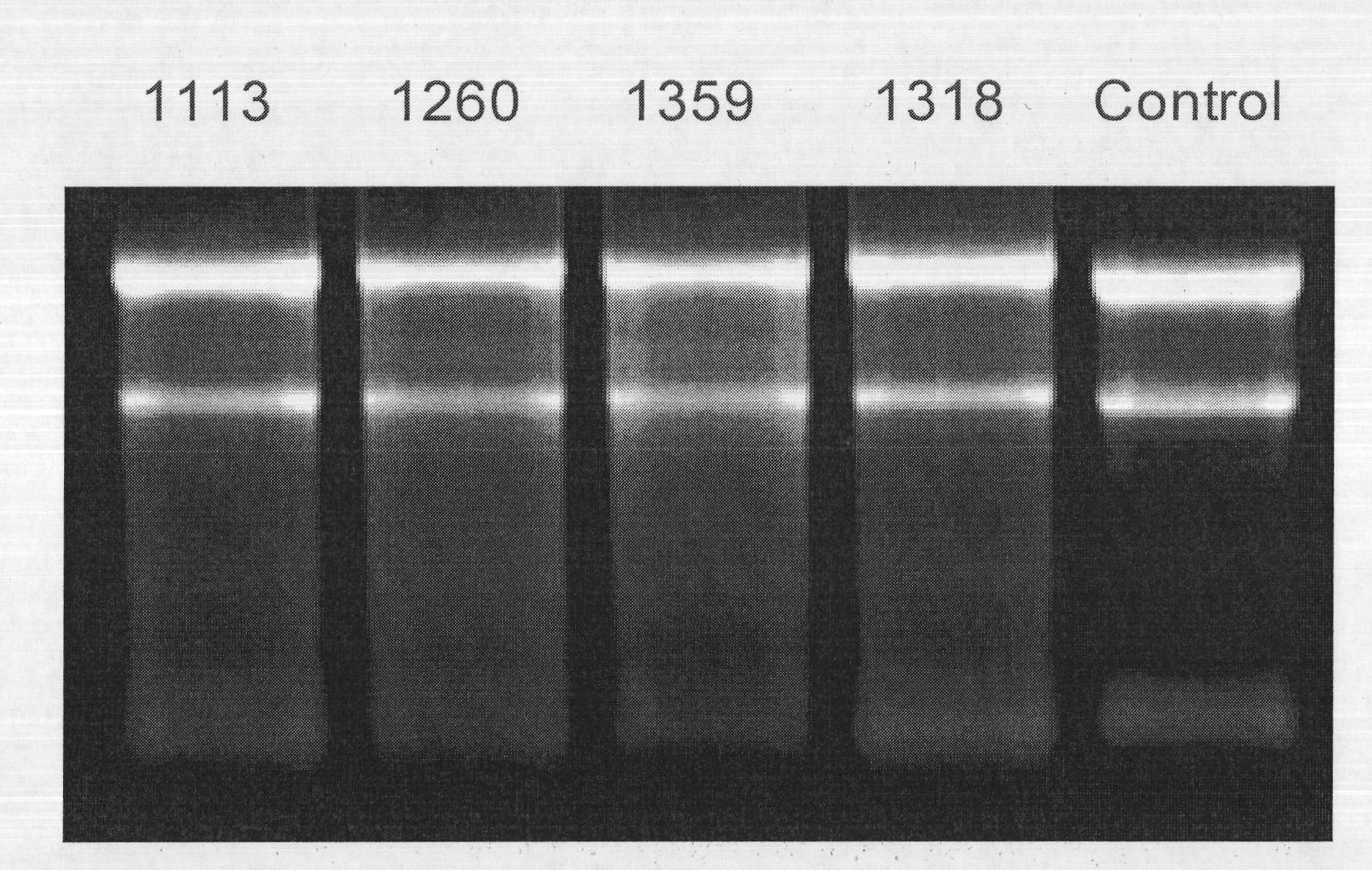
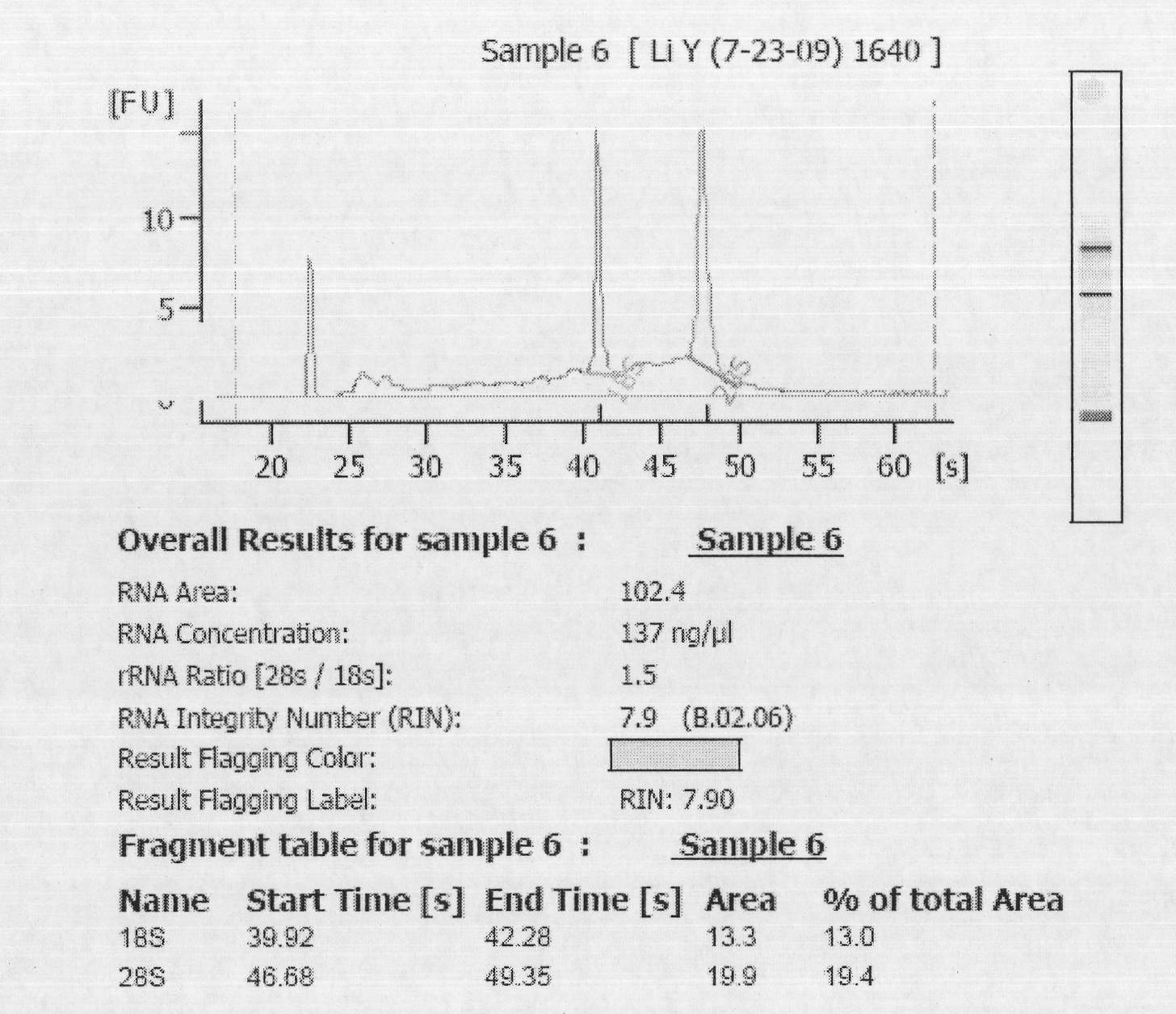
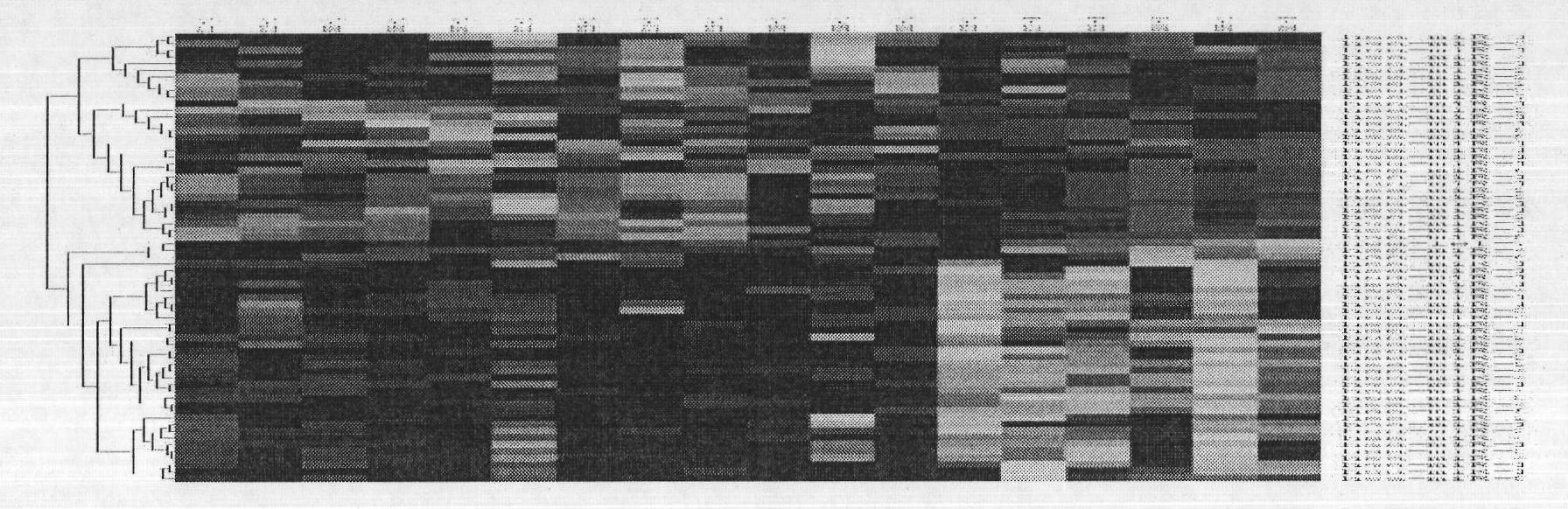
[0029] The present invention selects 30 cases of normal cervical epithelial tissues and 30 cervical cancer tissues. The source of normal cervical epithelium is collected from volunteers who come to the Obstetrics and Gynecology Hospital Affiliated to Zhejiang University School of Medicine for cervical disease screening. Cervical cancer patients treated (23 cases of squamous cell carcinoma, 4 cases of adenosquamous cell carcinoma, 3 cases of adenocarcinoma). All specimens were biopsied tissues under colposcopy, and each specimen was divided into two parts, one was quick-frozen in liquid nitrogen and then stored in liquid nitrogen, and the other was fixed in 4% formaldehyde and embedded in stone vinegar. Liquid nitrogen cryopreserved tissue was used for Micro RNA chip inspection to screen internal reference molecules and to ve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com