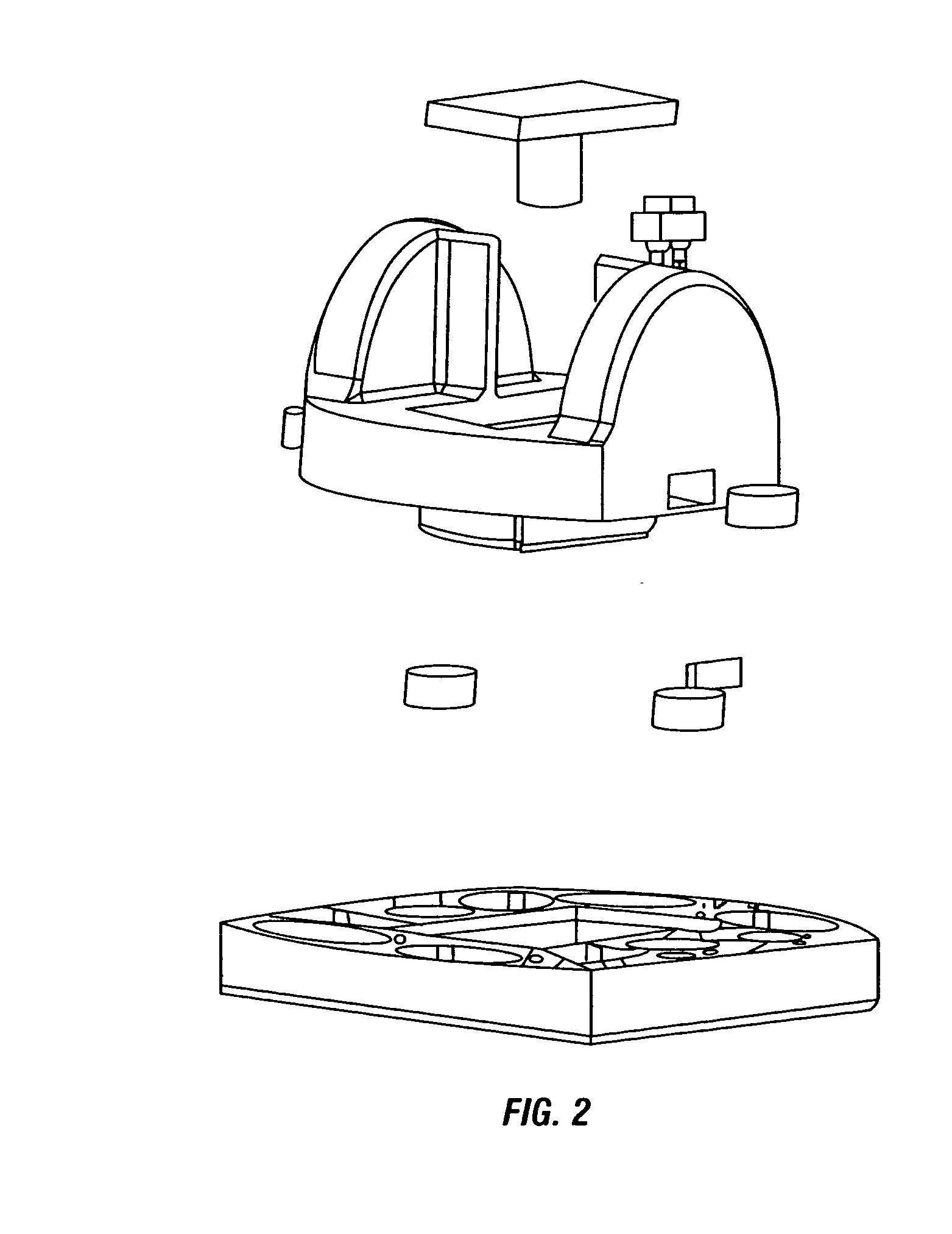
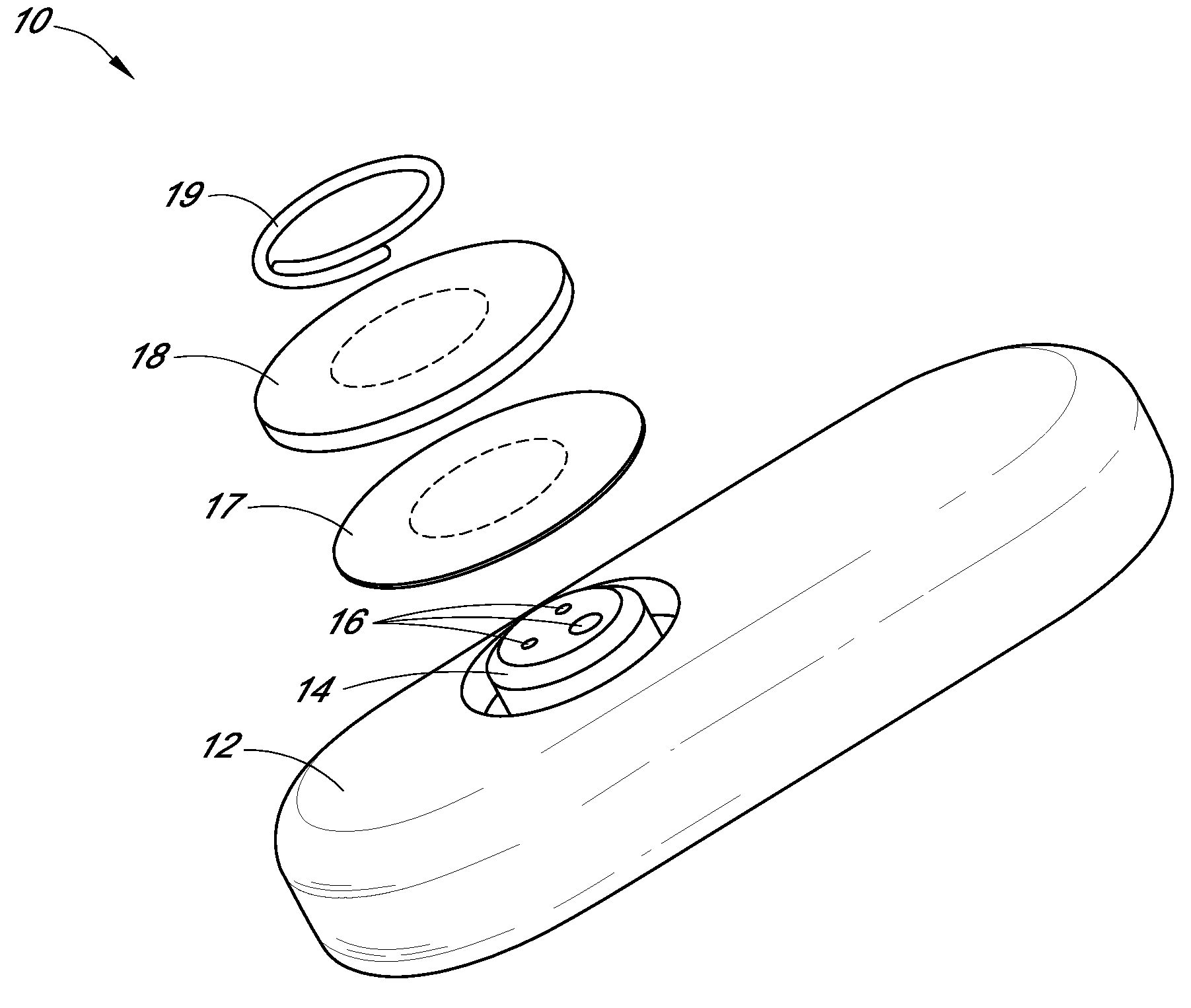
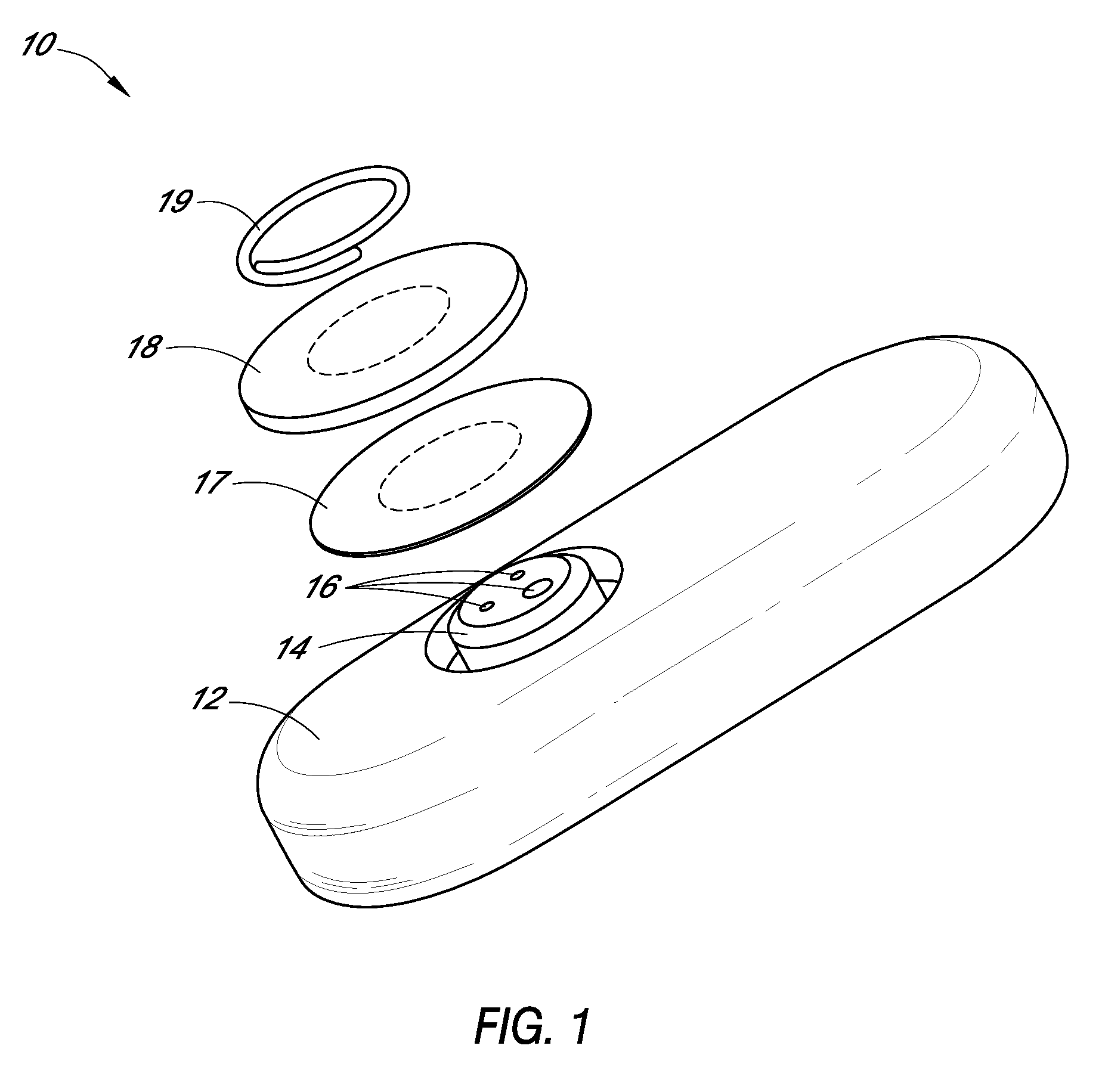
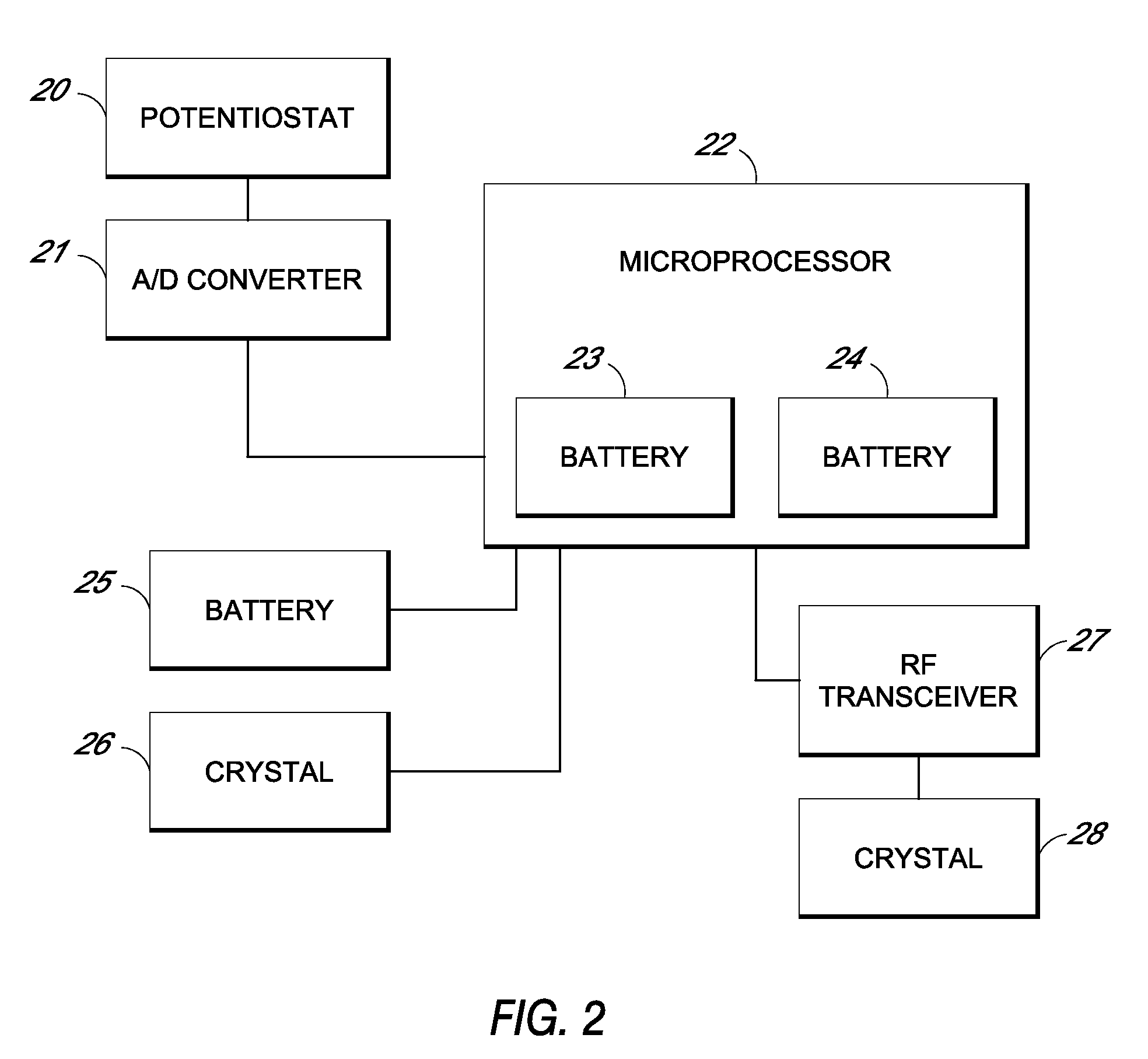
Patents
Literature
112 results about "Reference analysis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
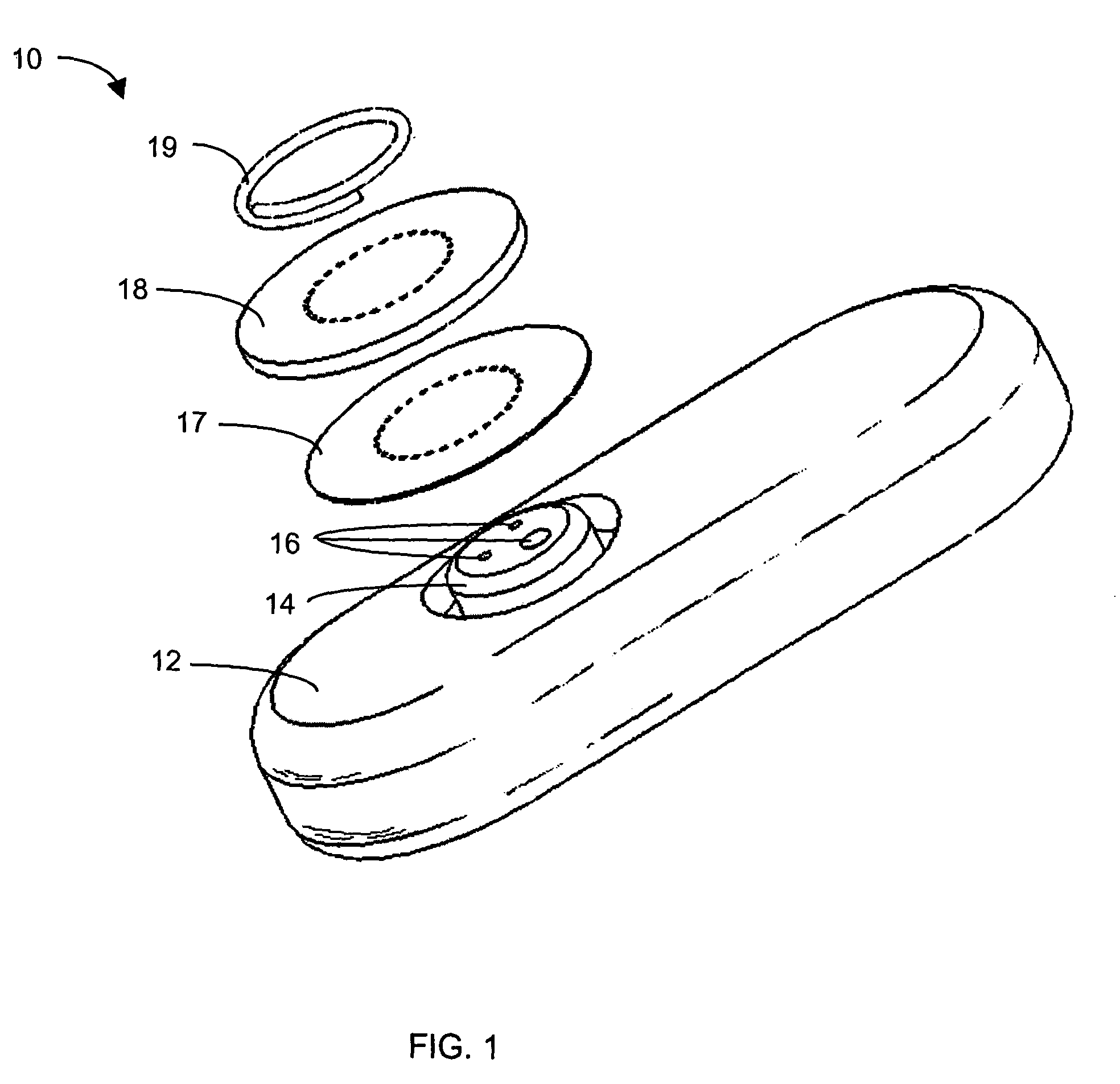
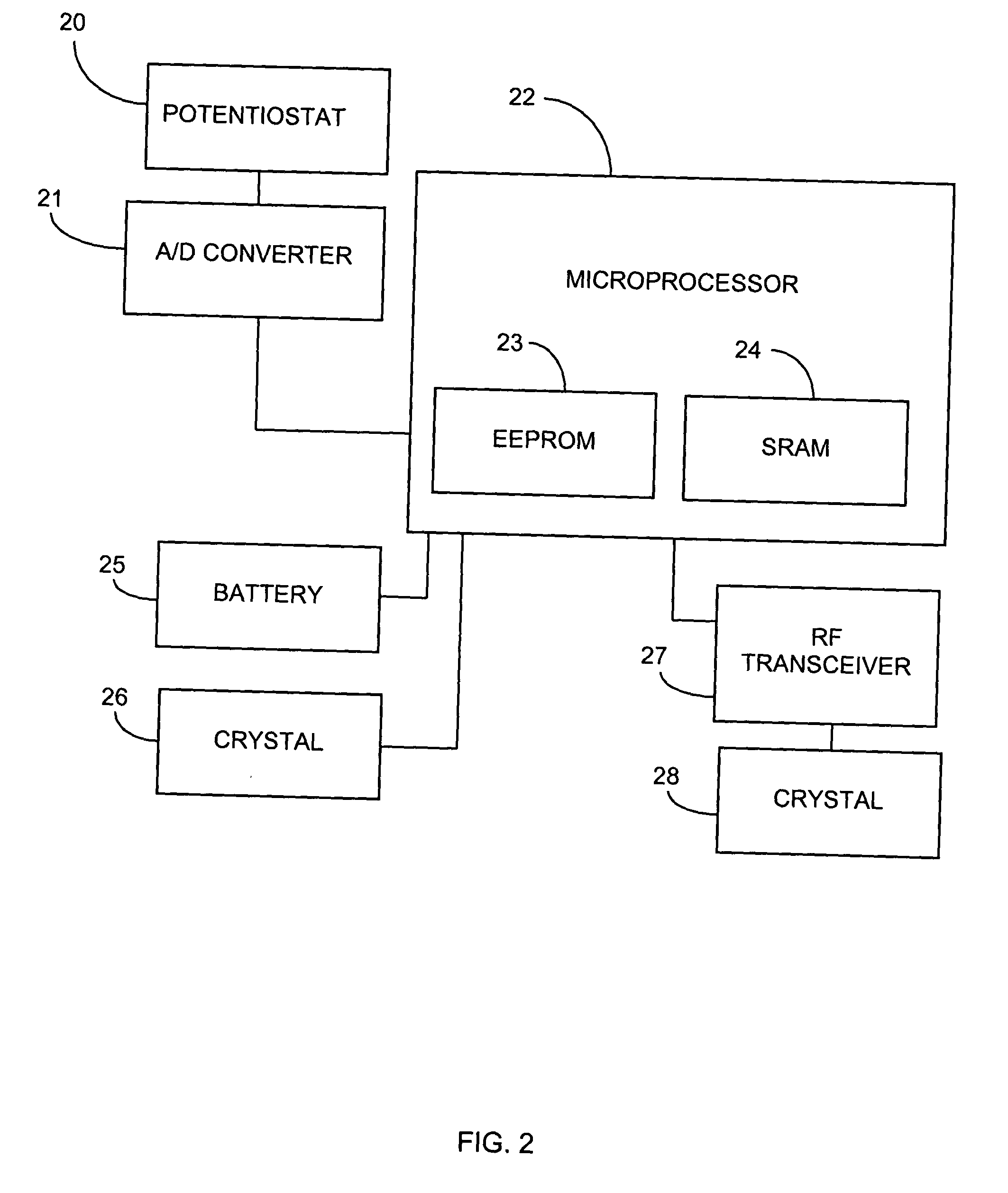
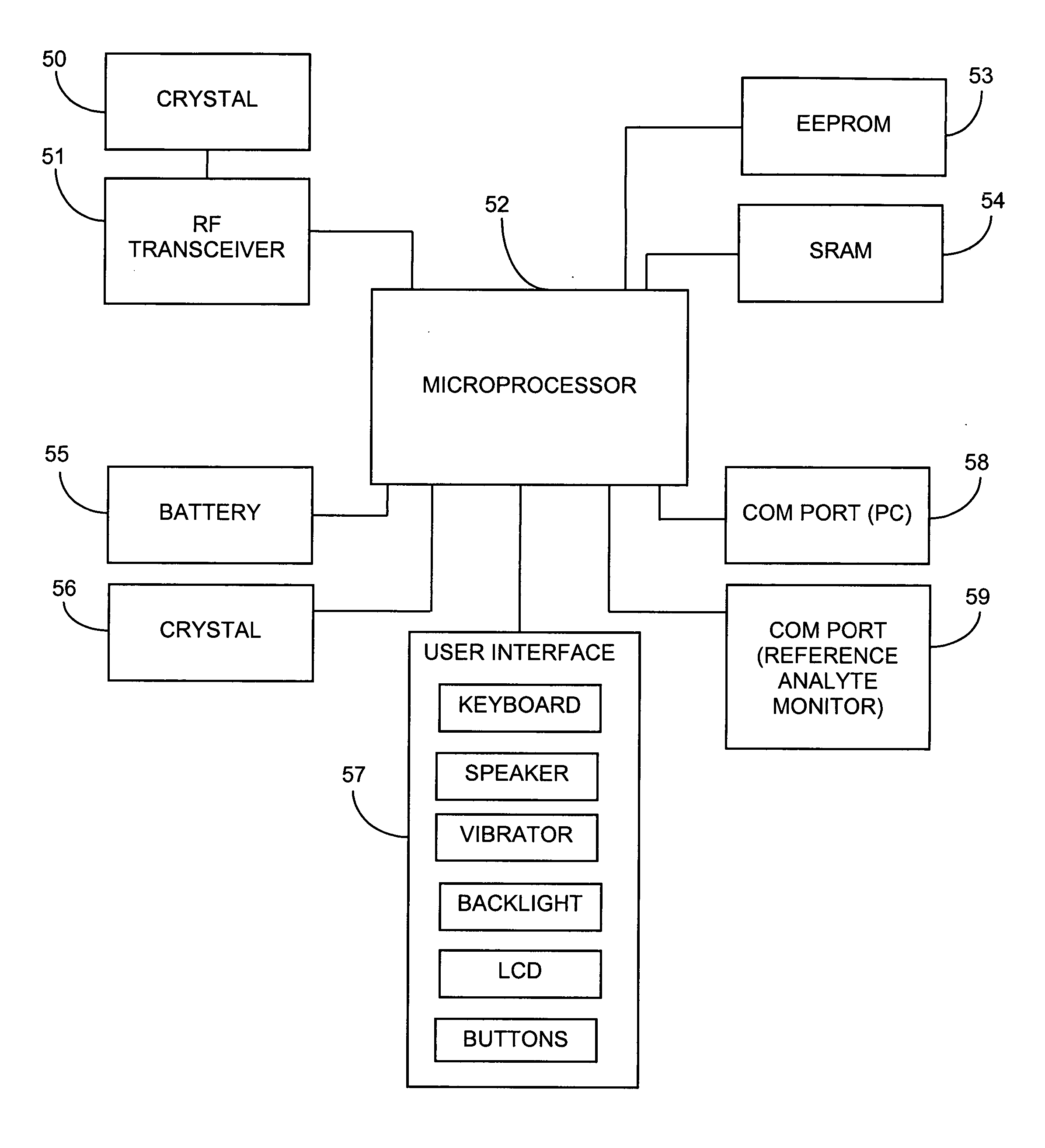
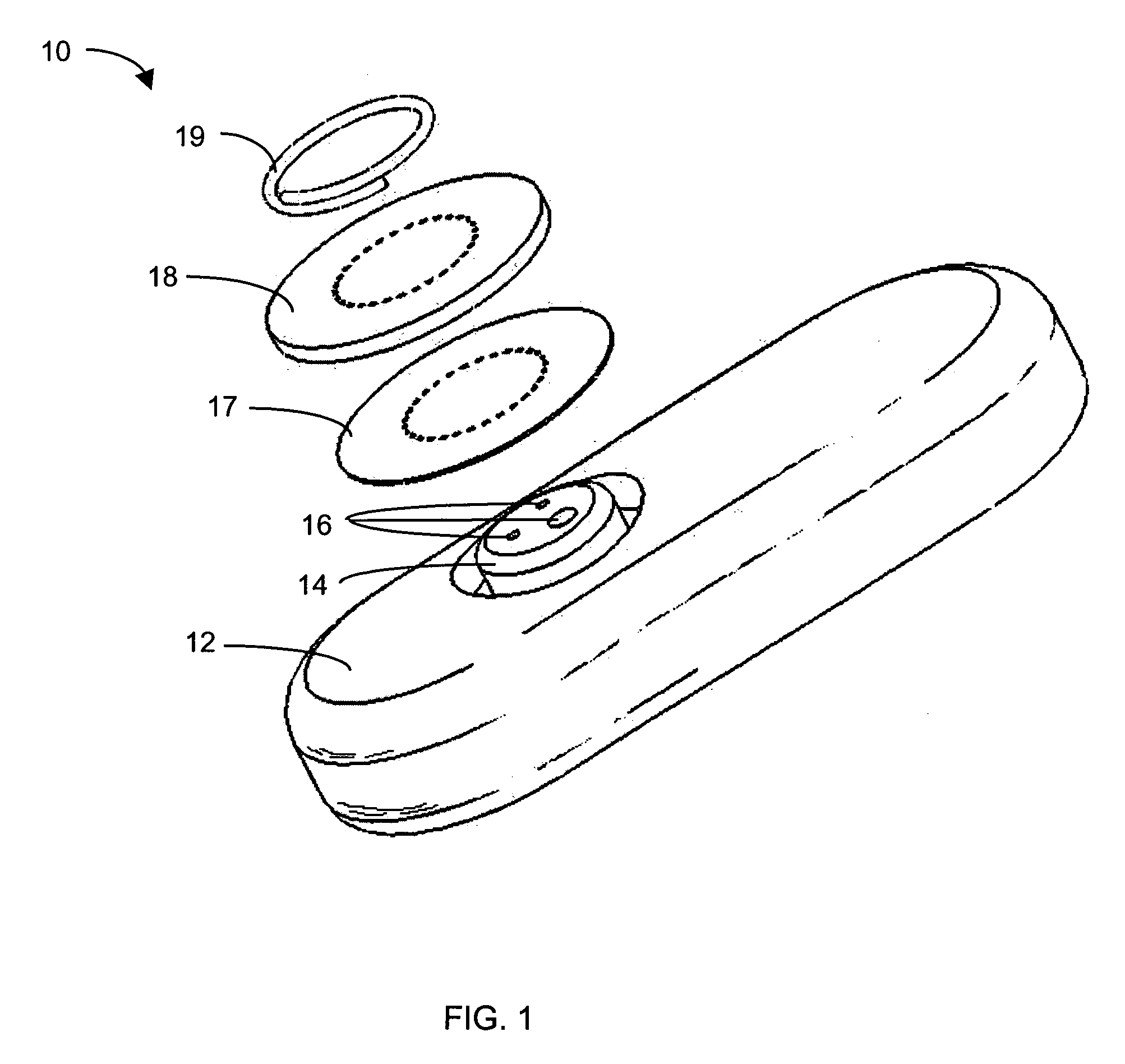
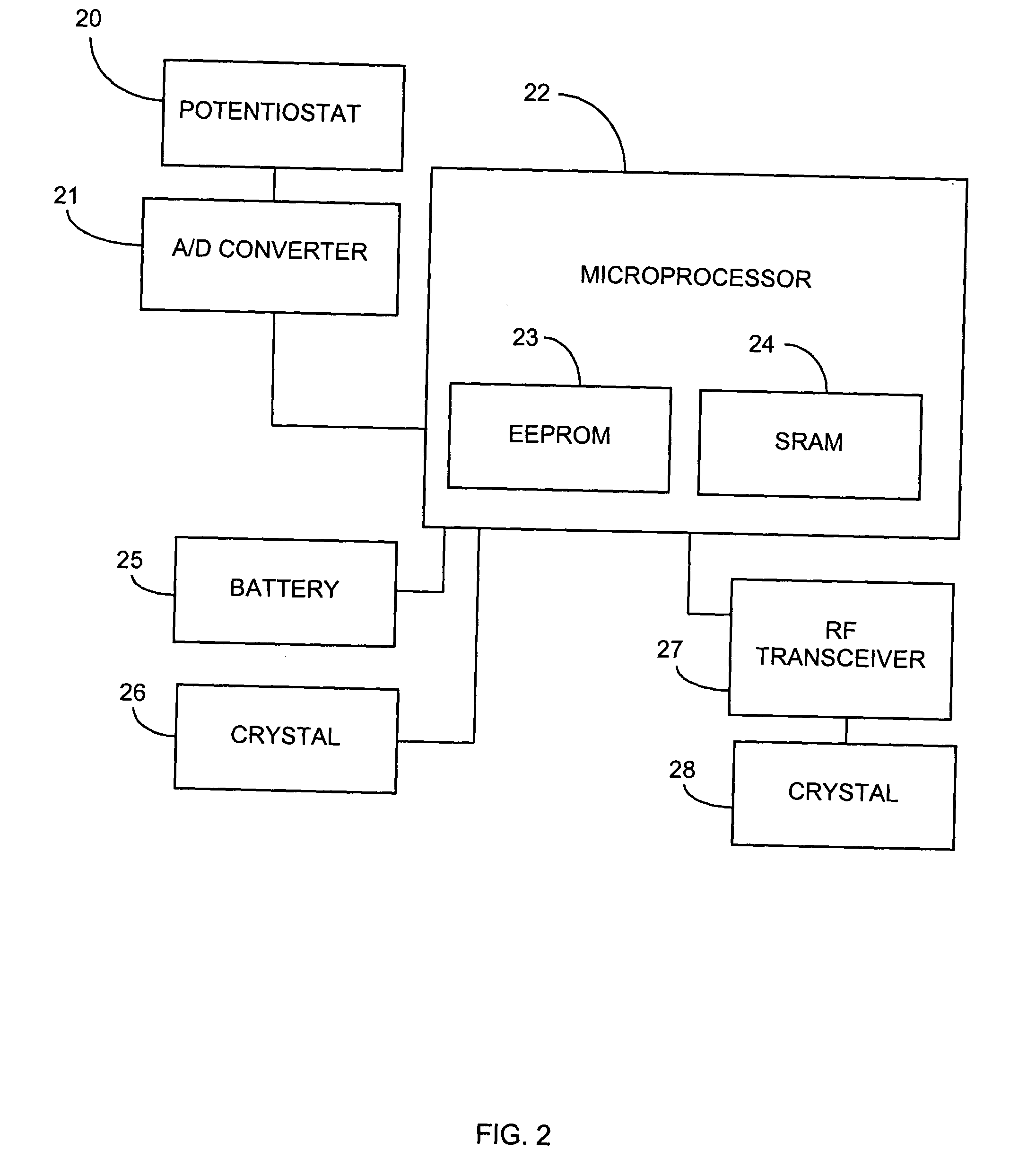
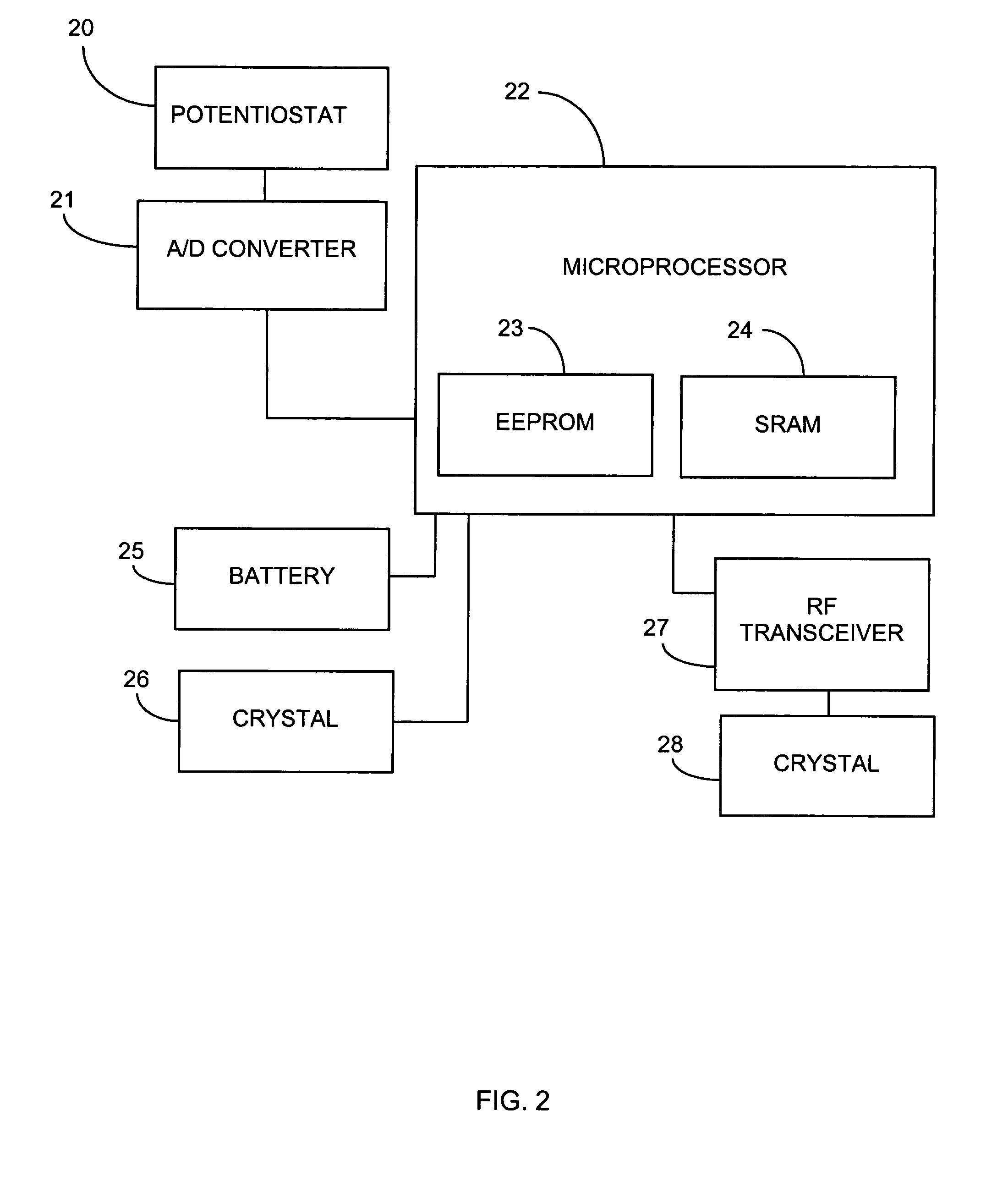
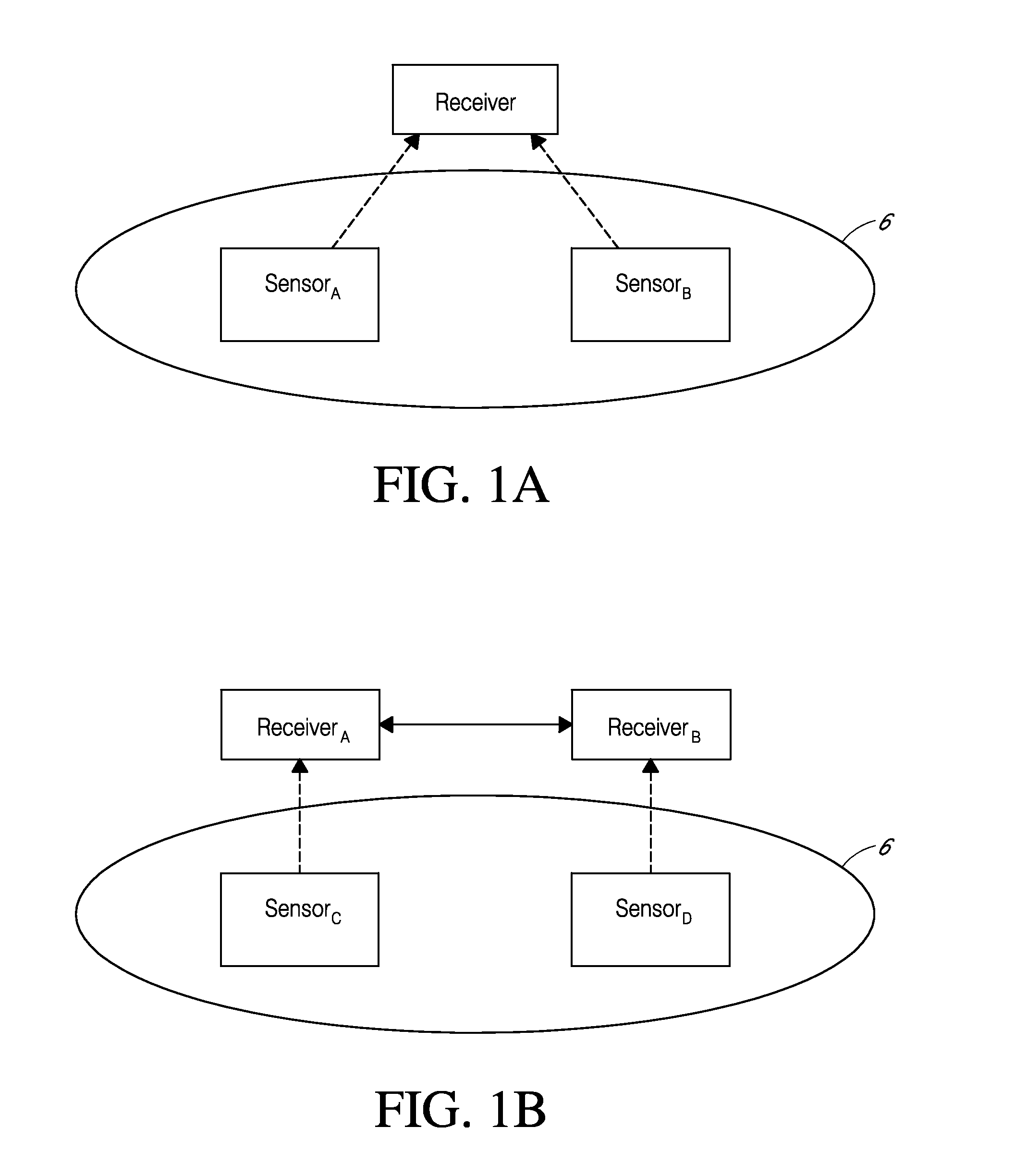
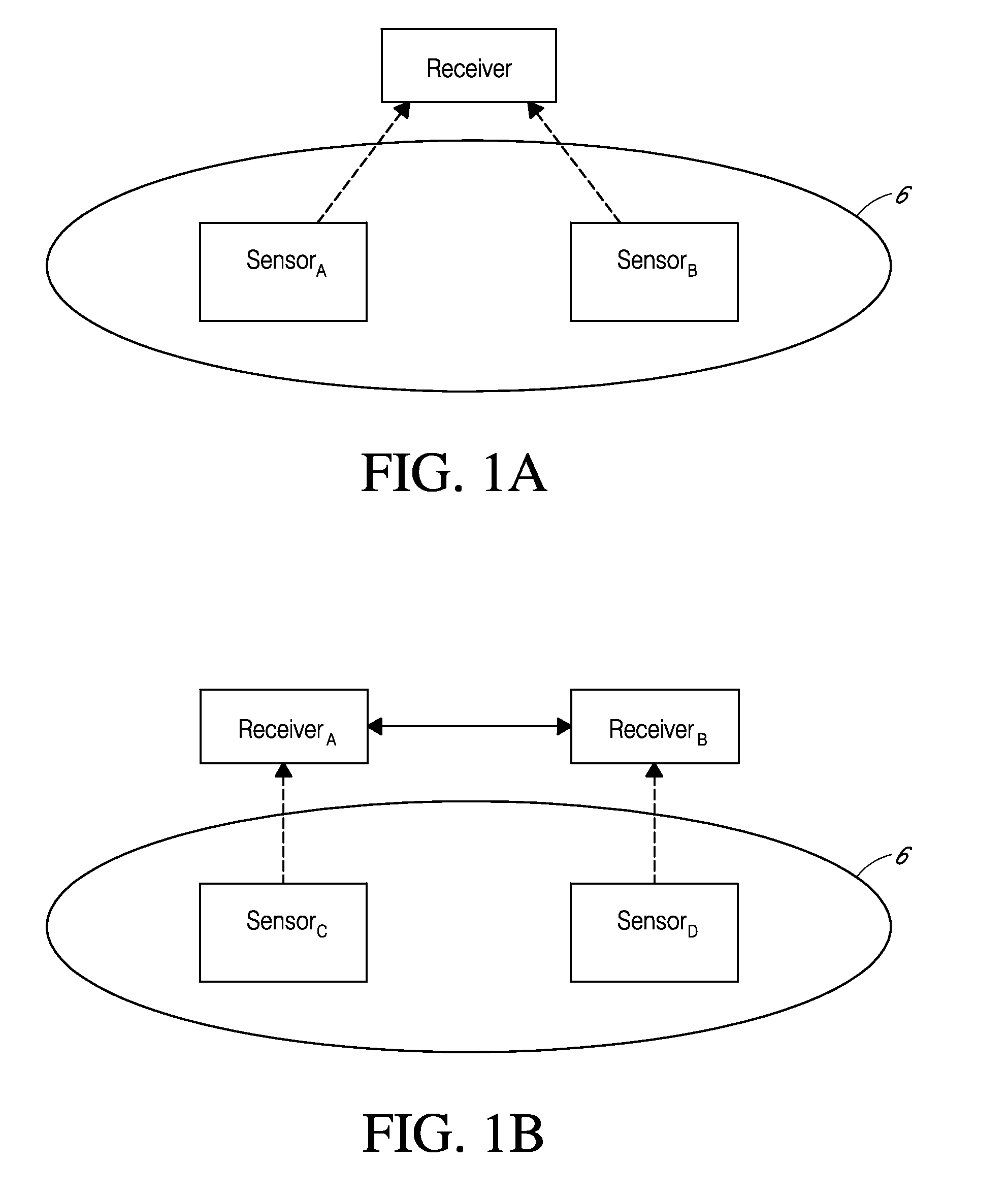
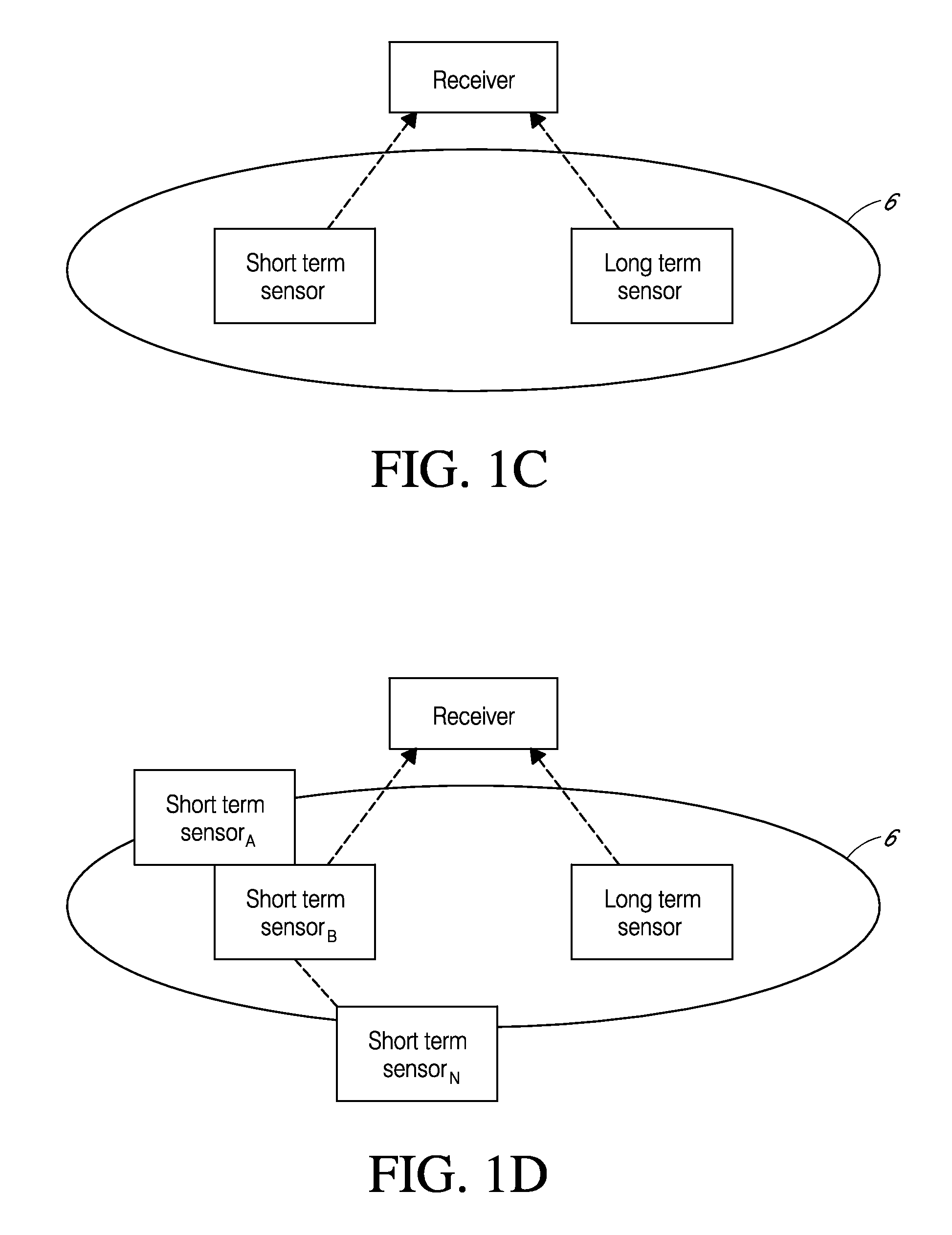
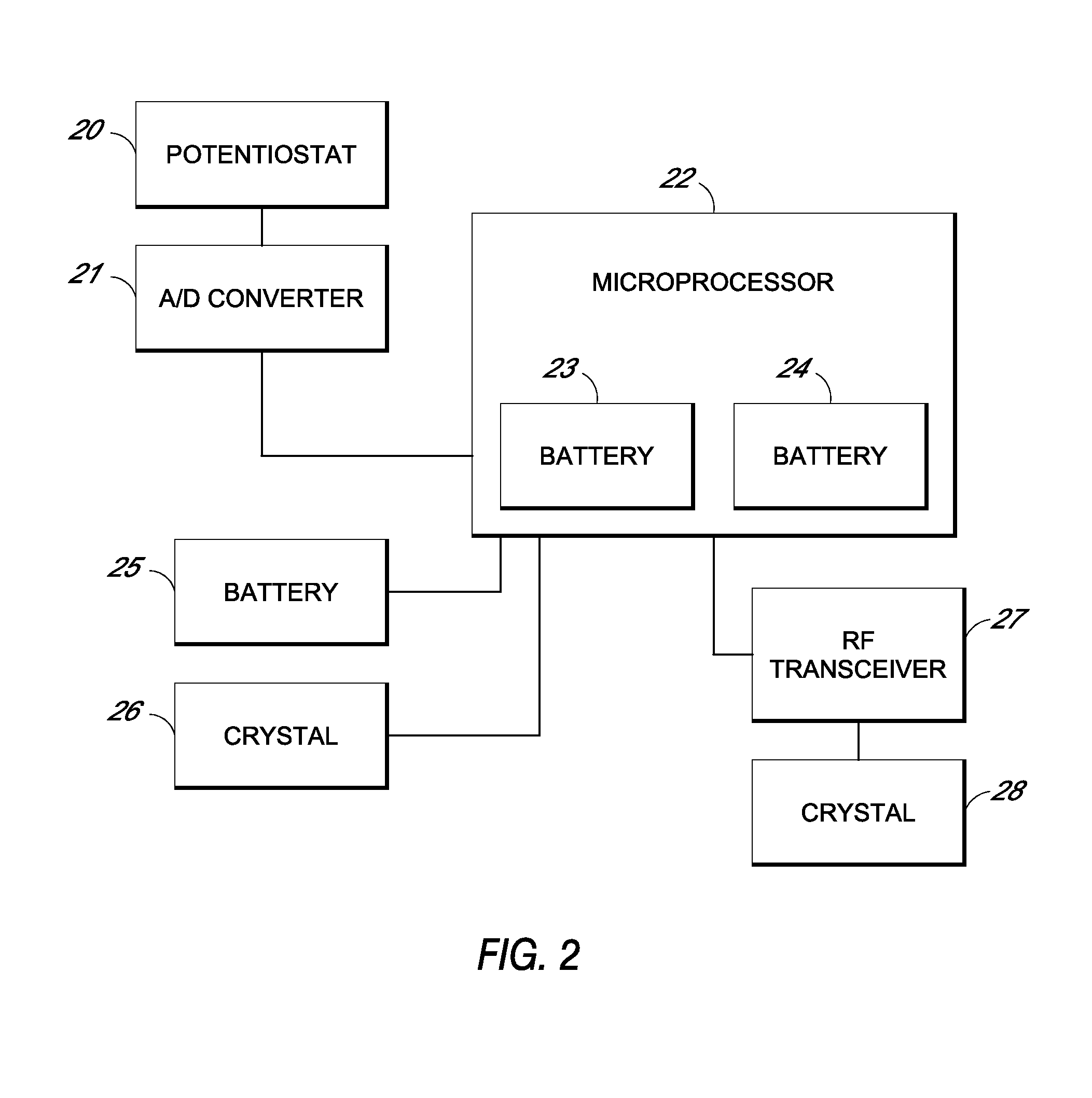
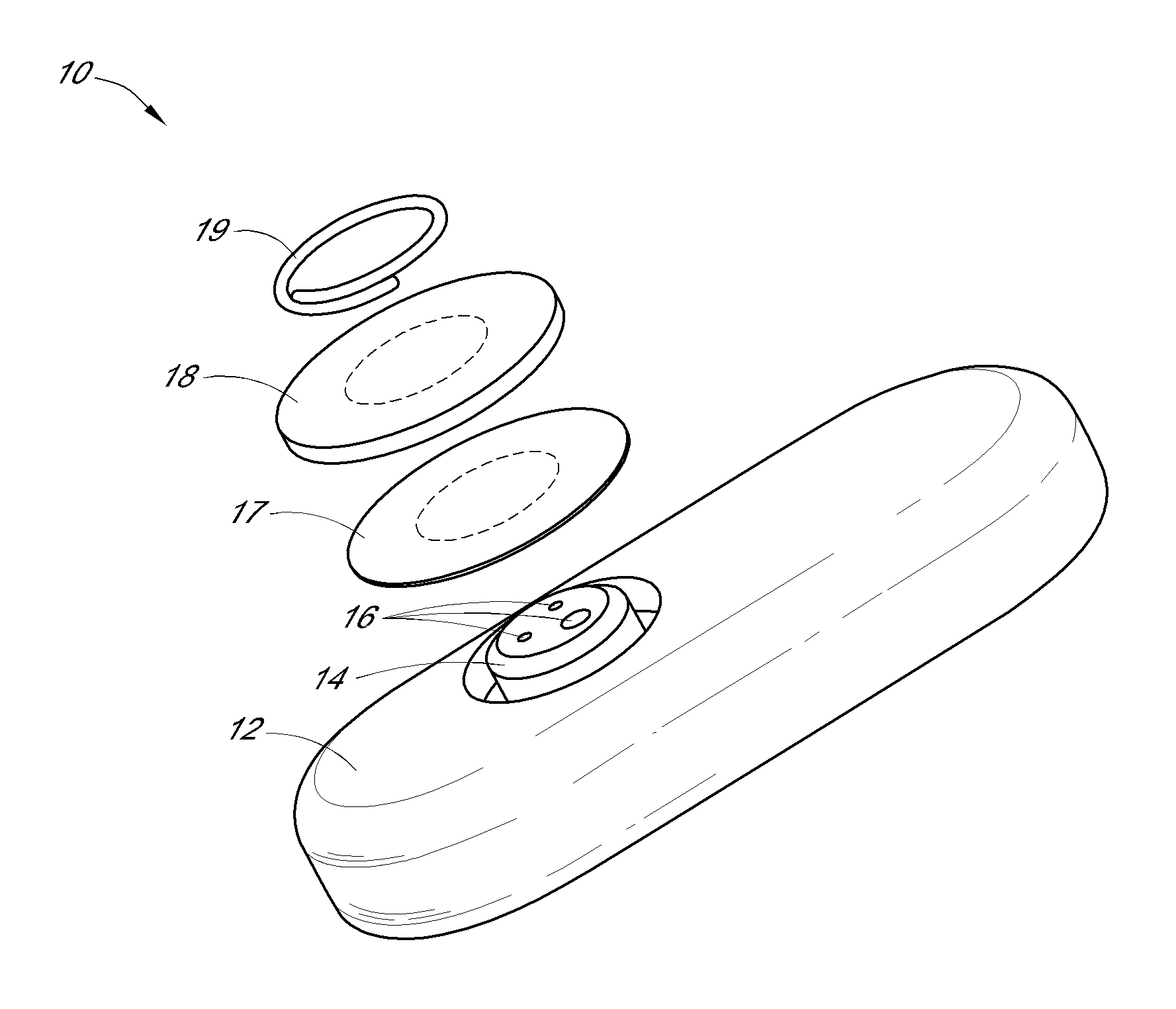
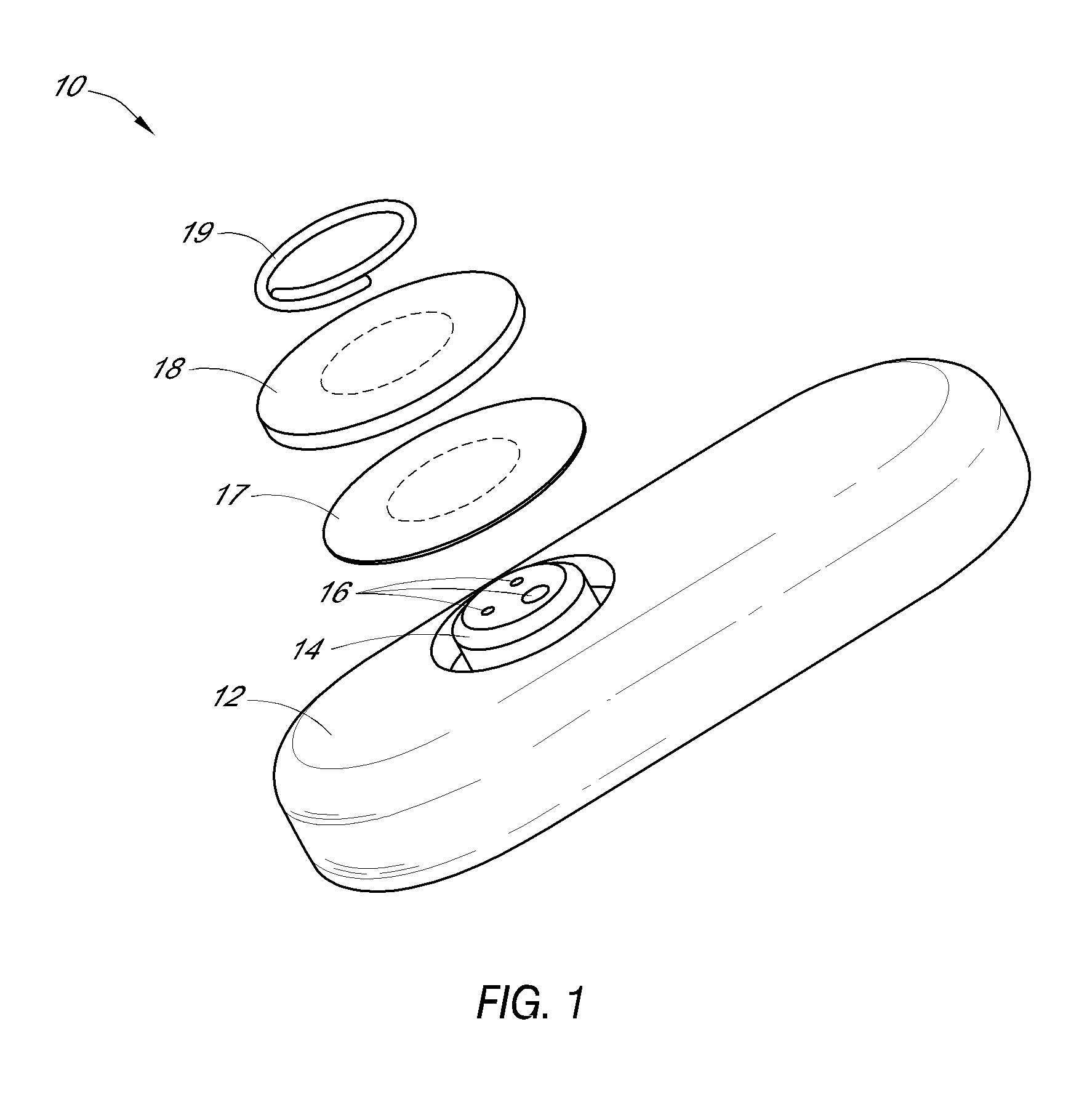
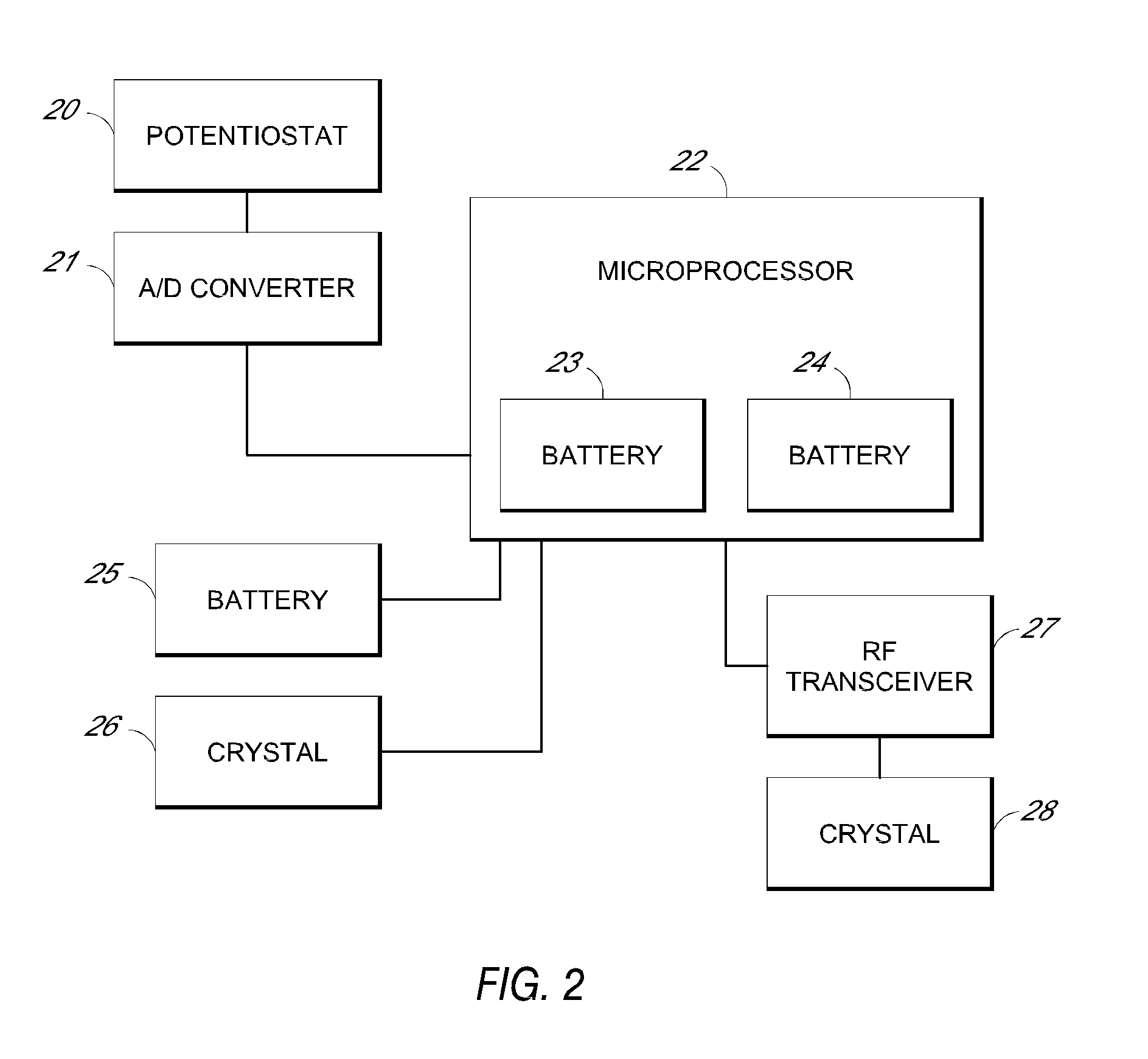
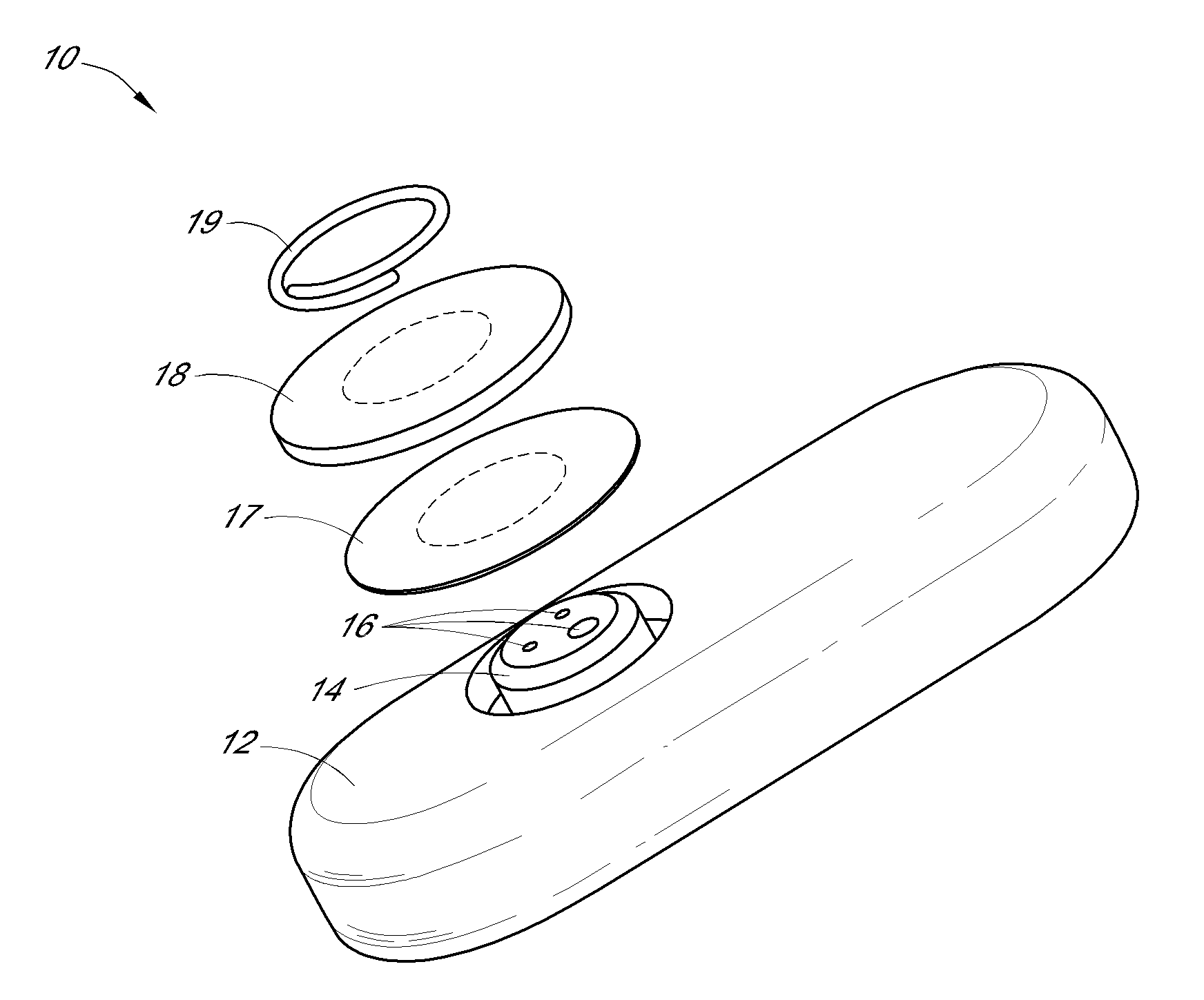
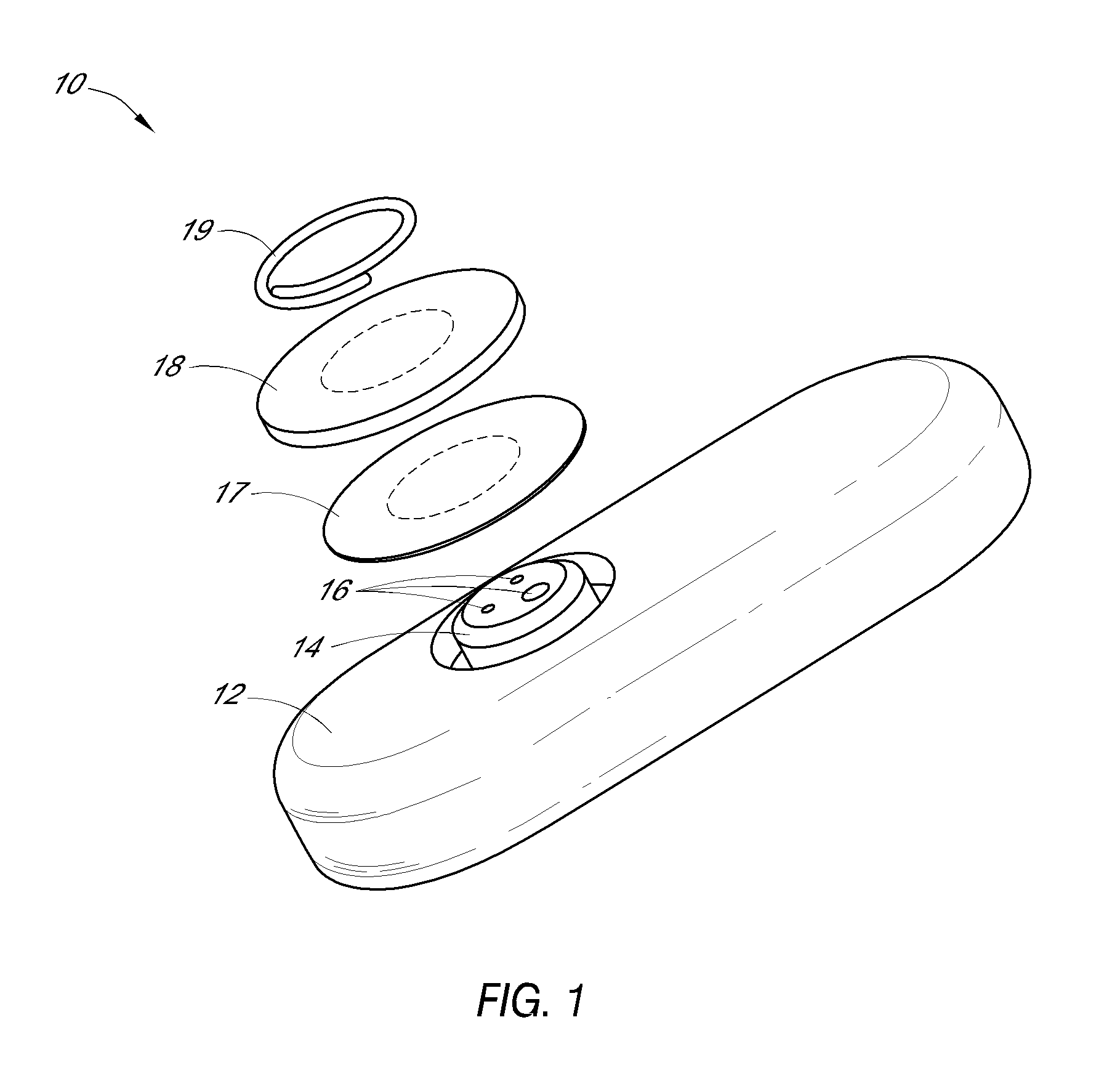
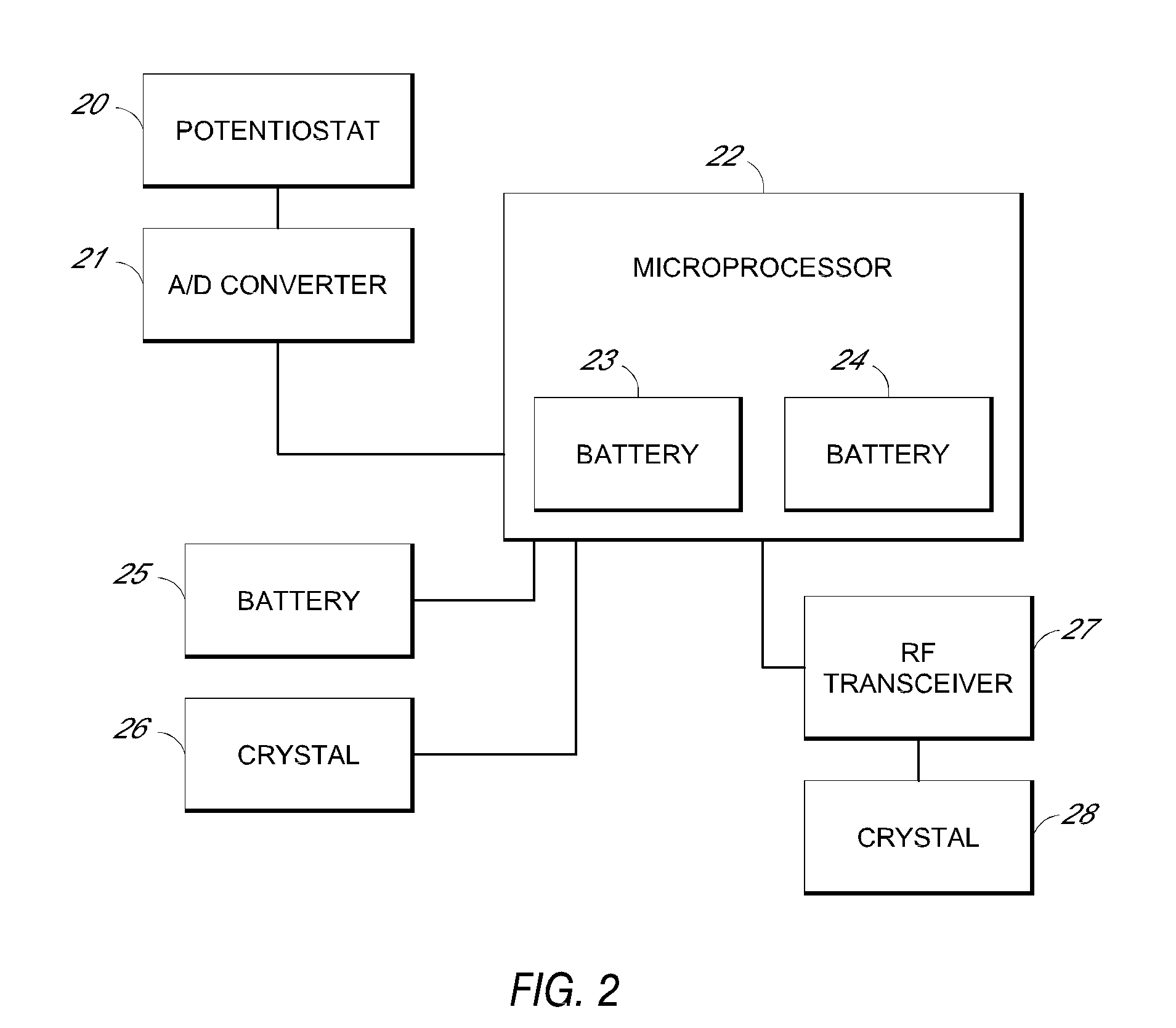
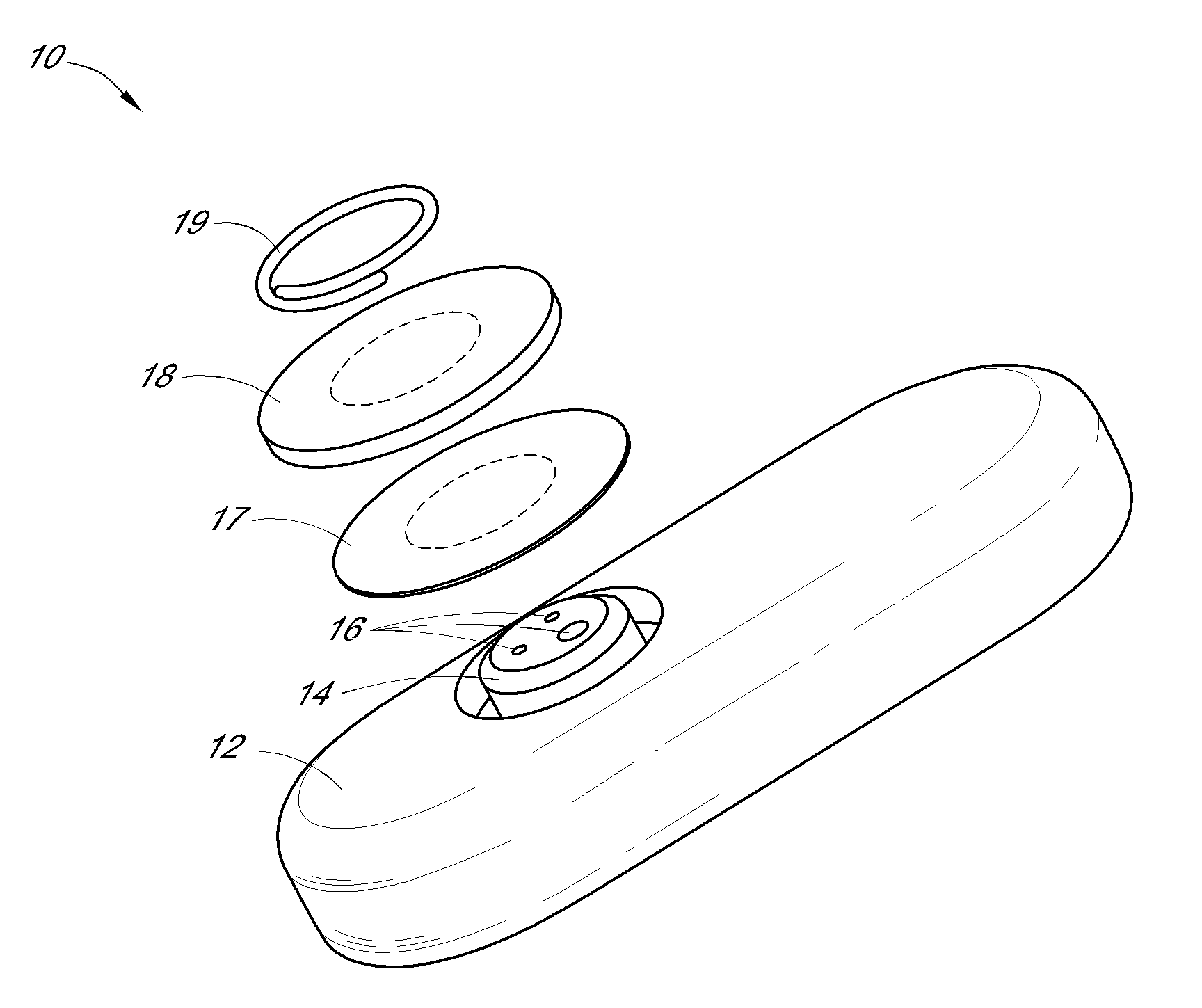
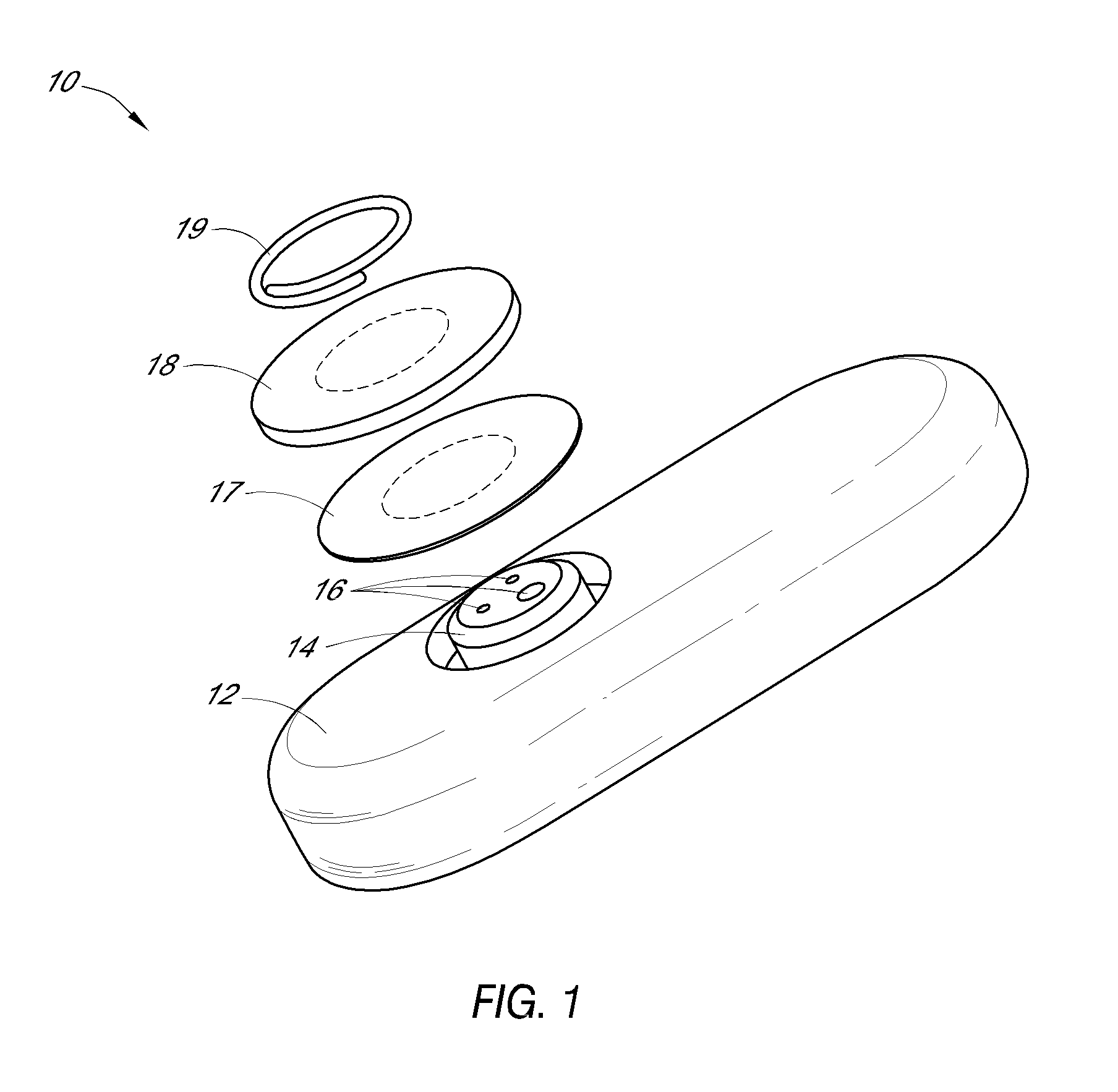
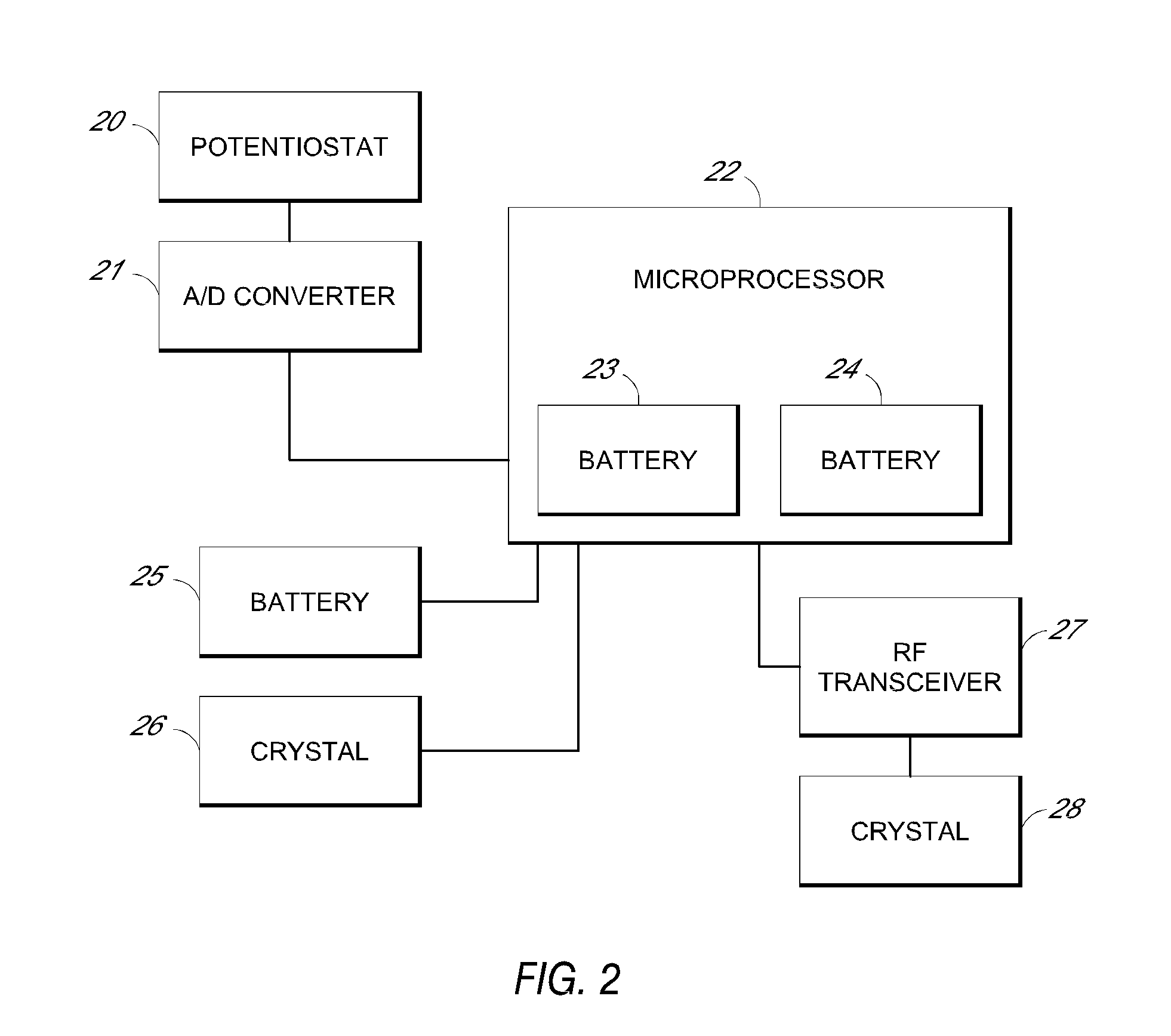
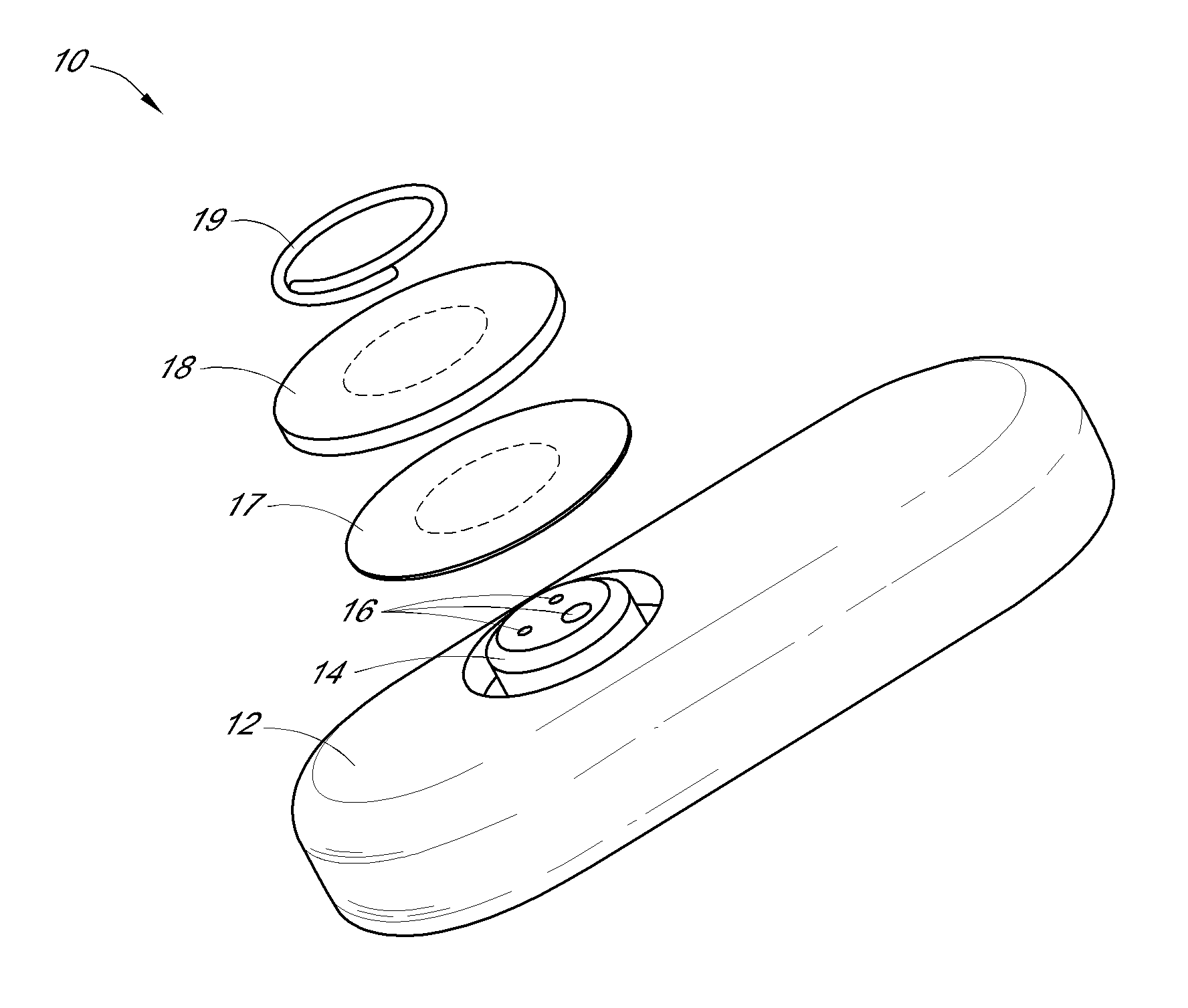
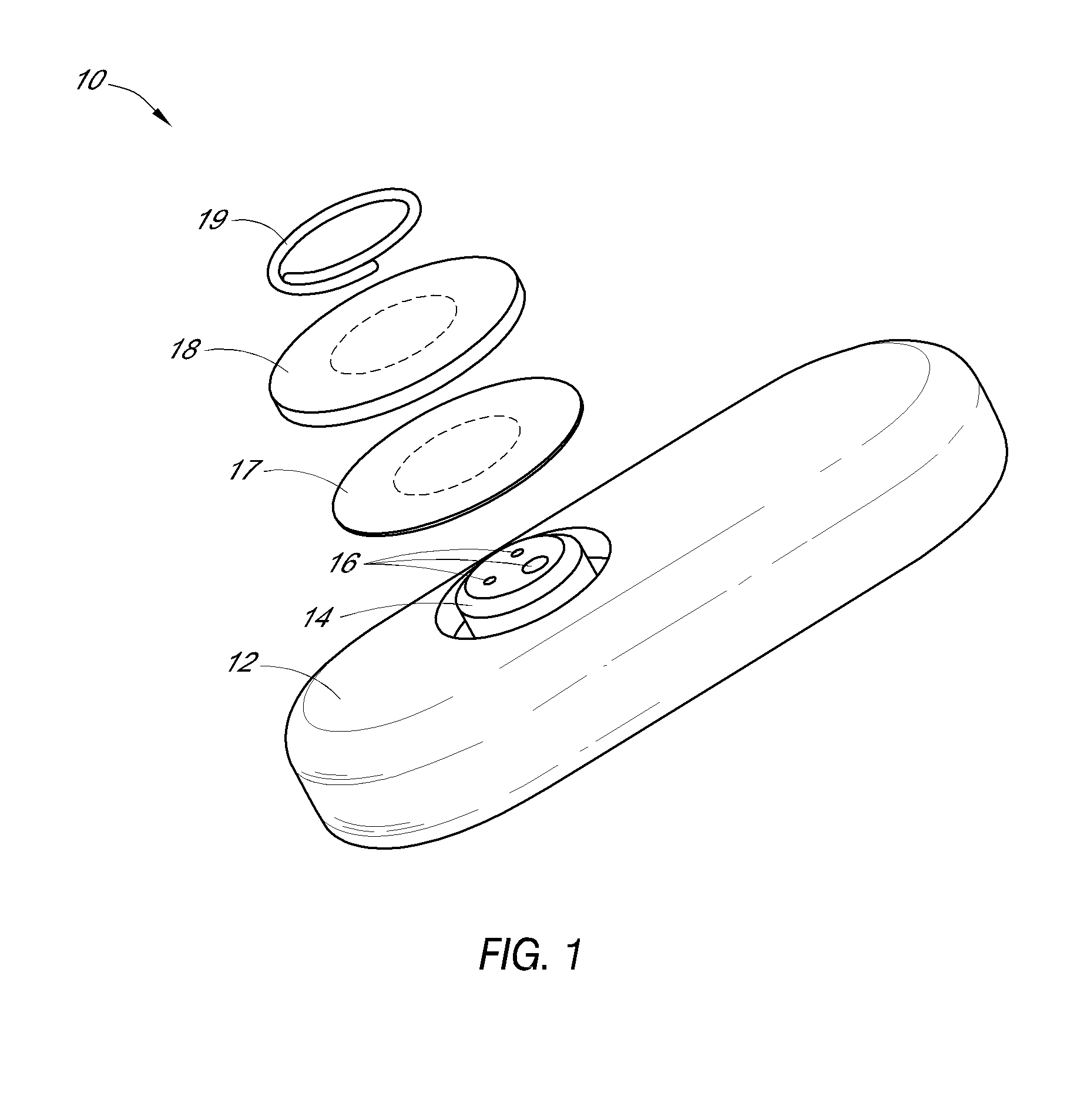
System and methods for processing analyte sensor data
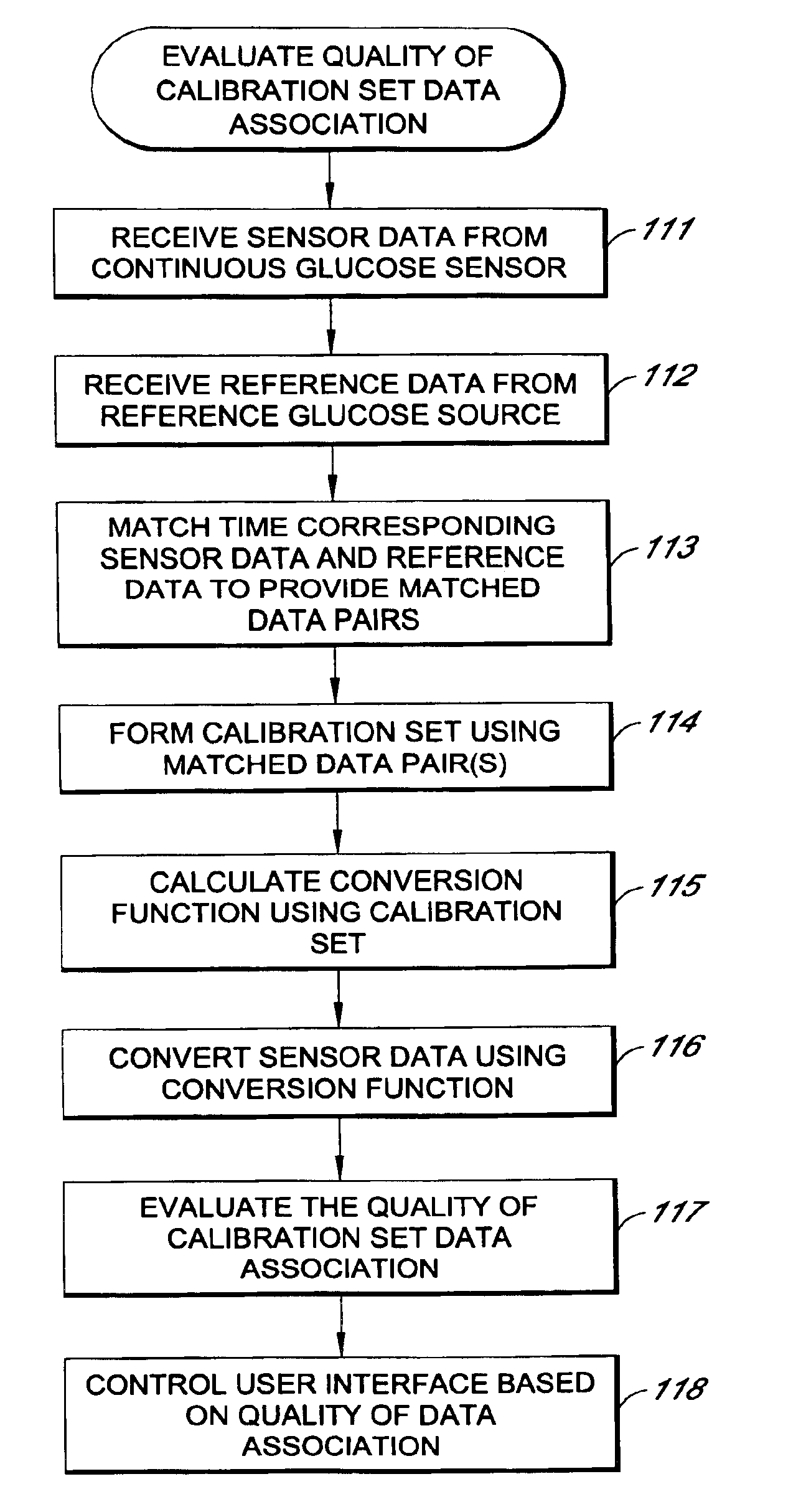
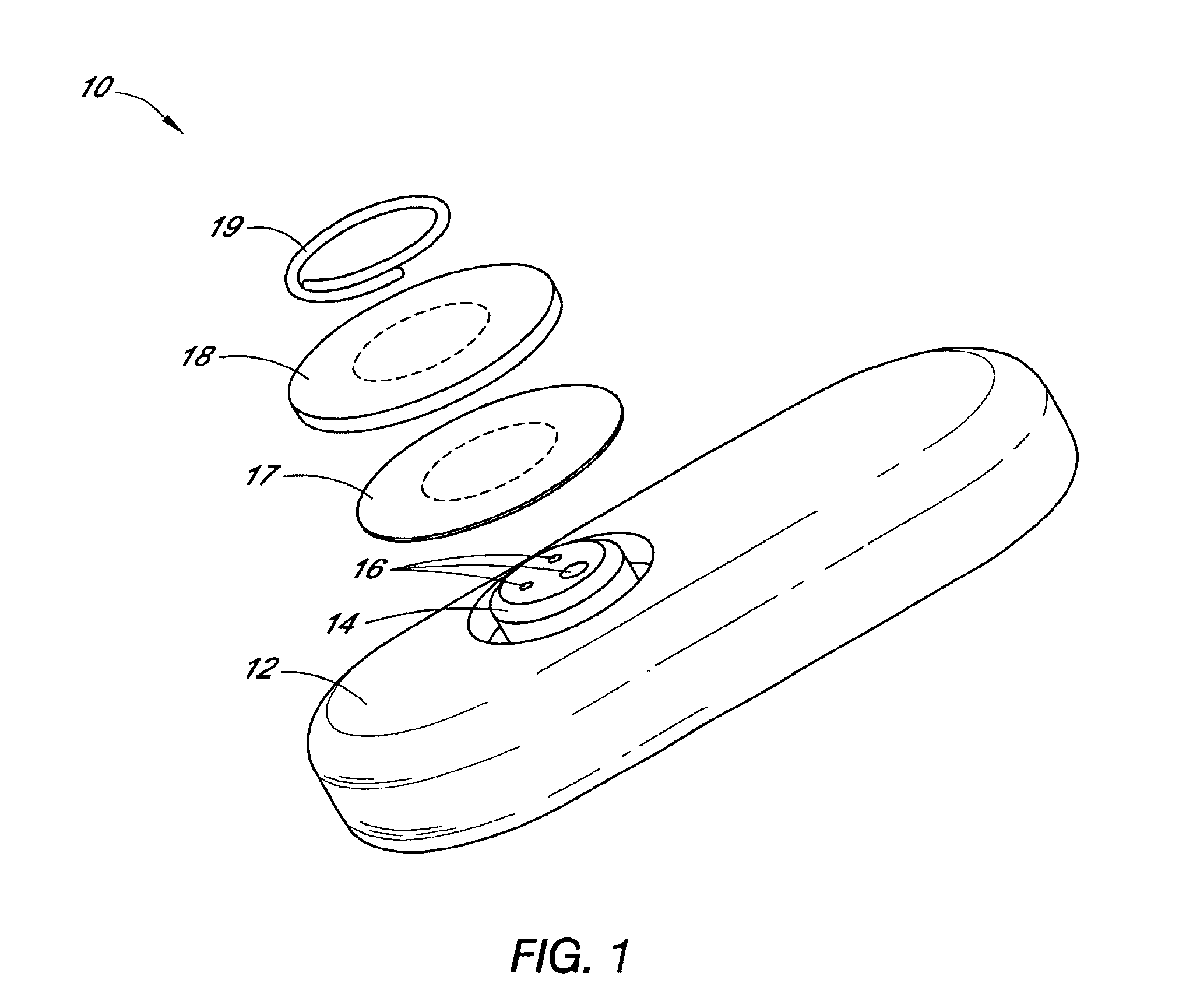
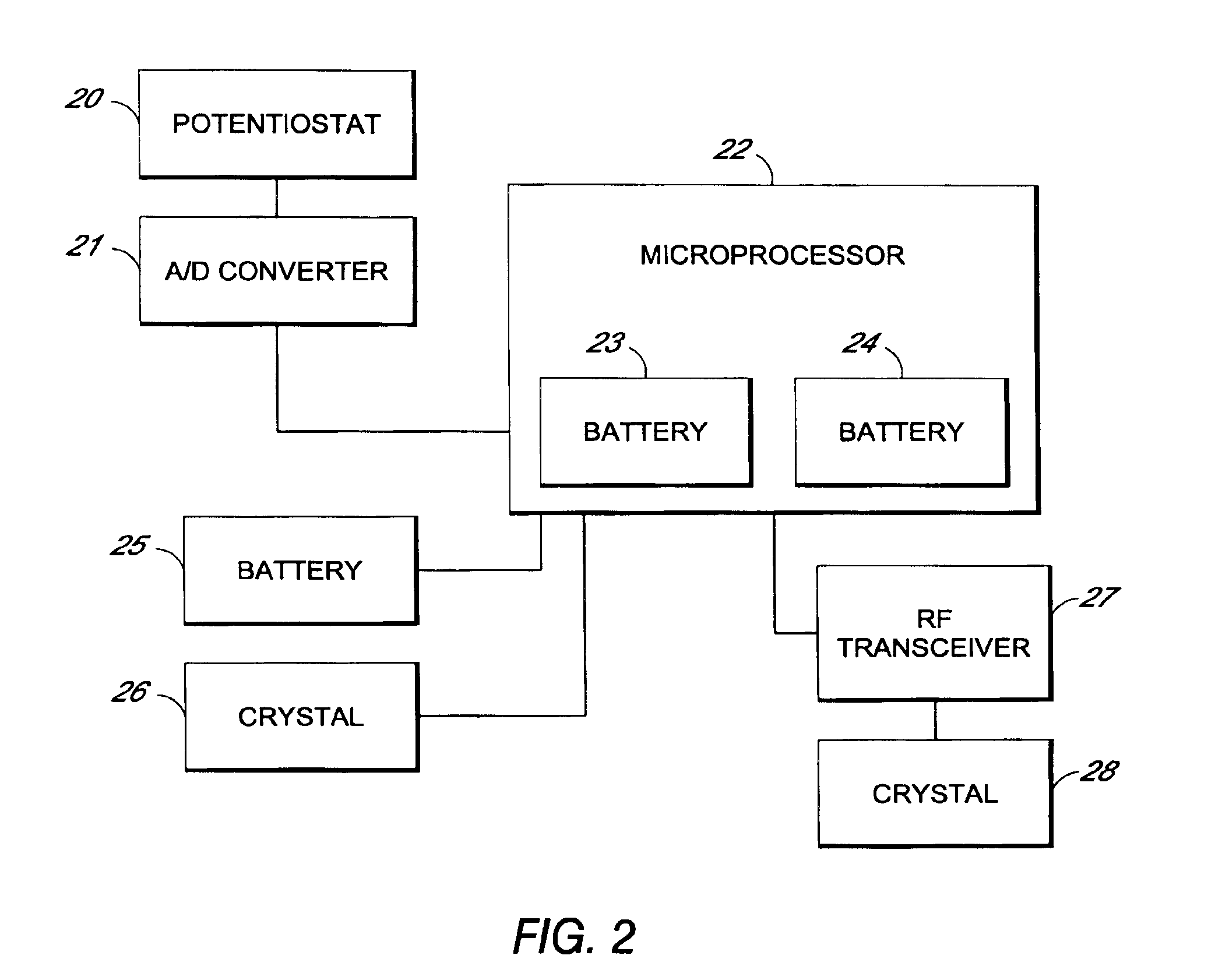
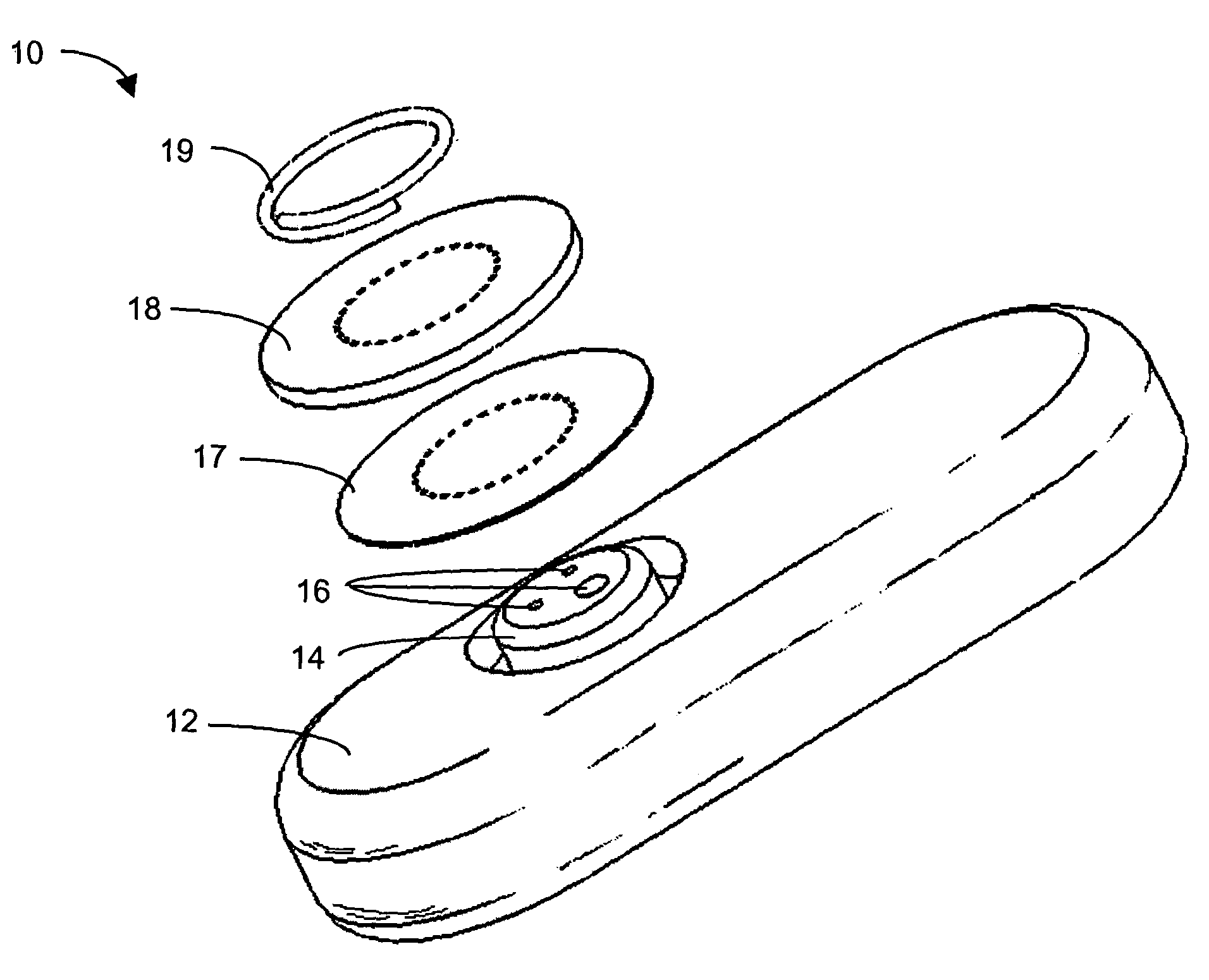
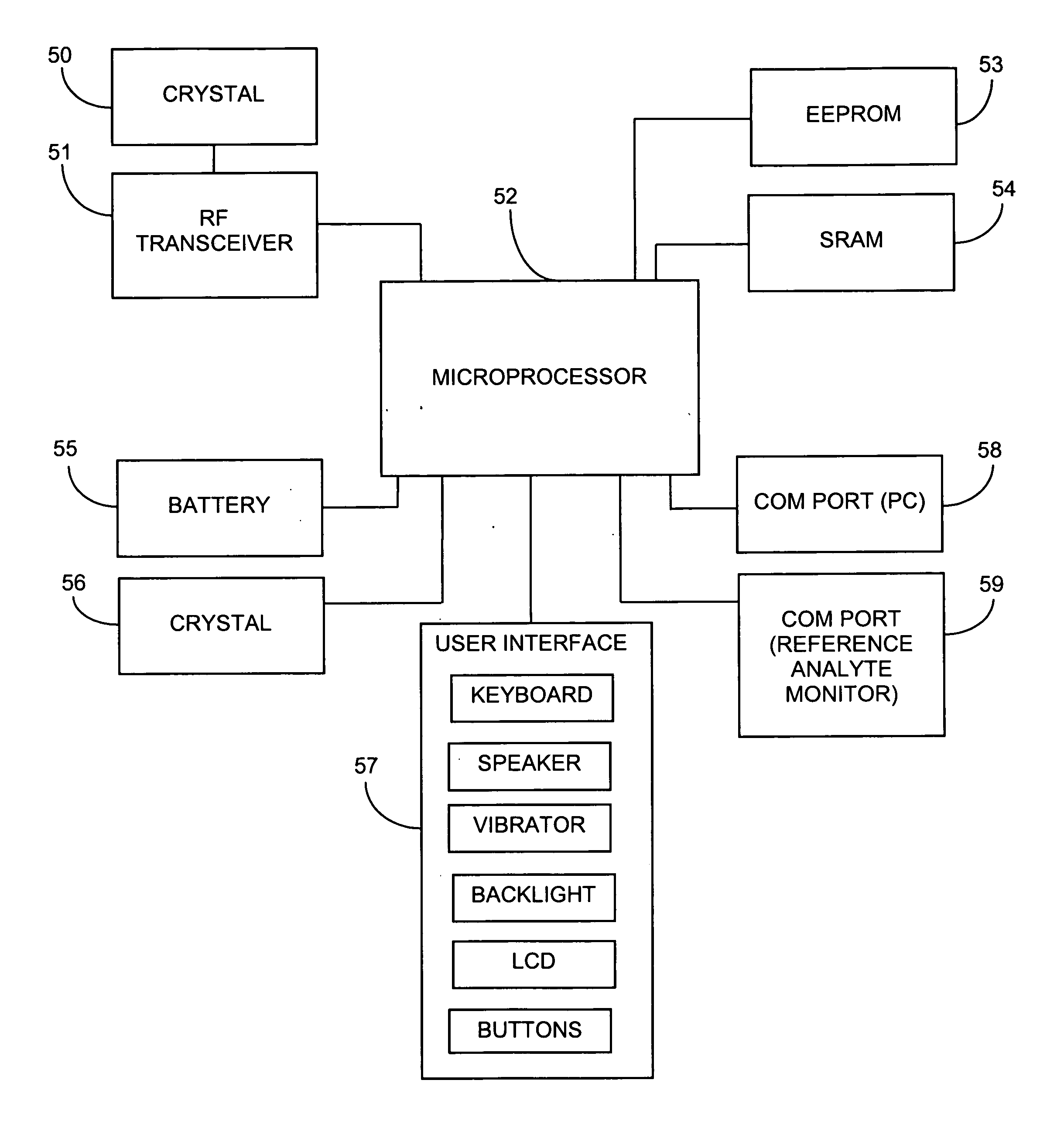
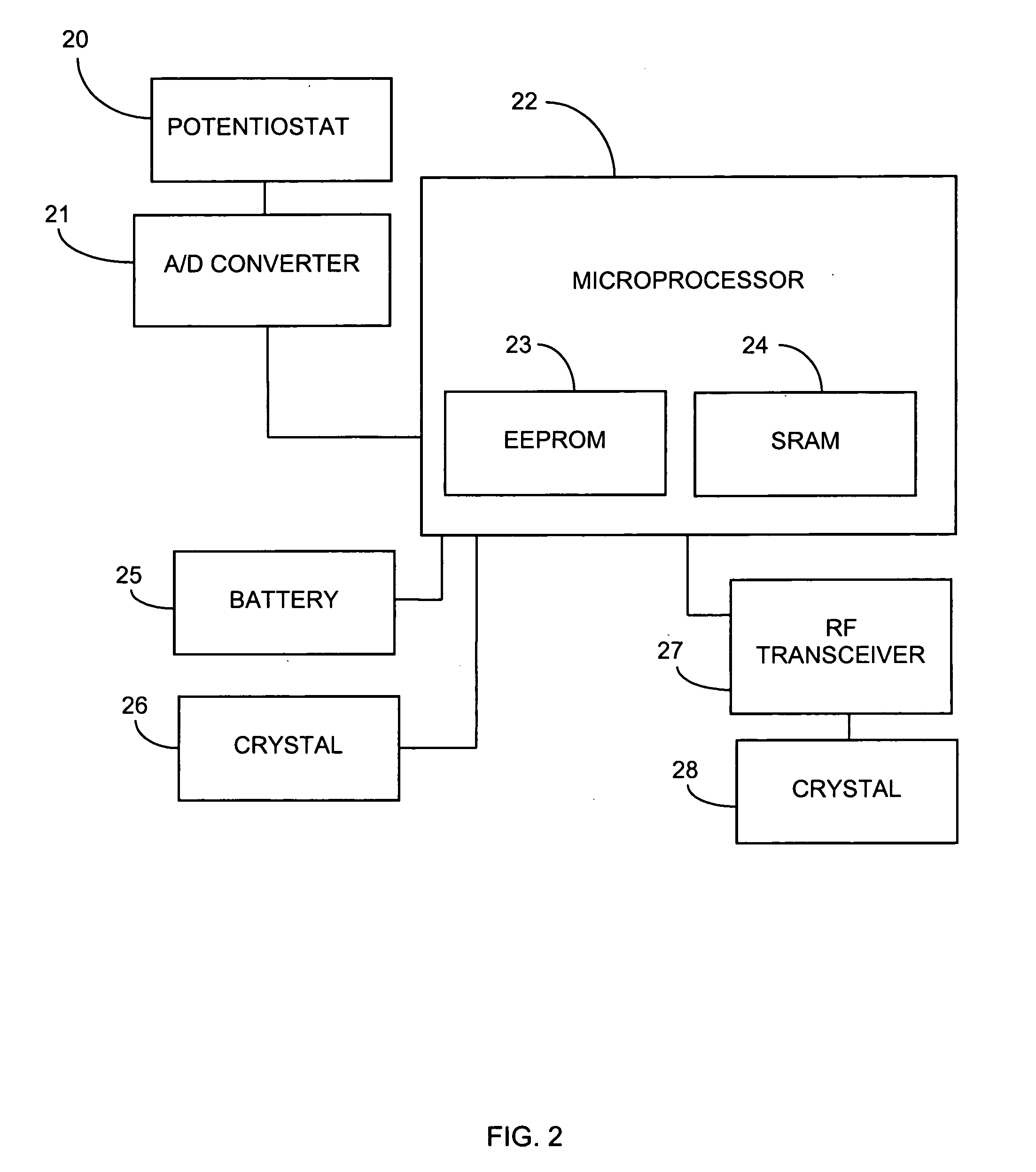
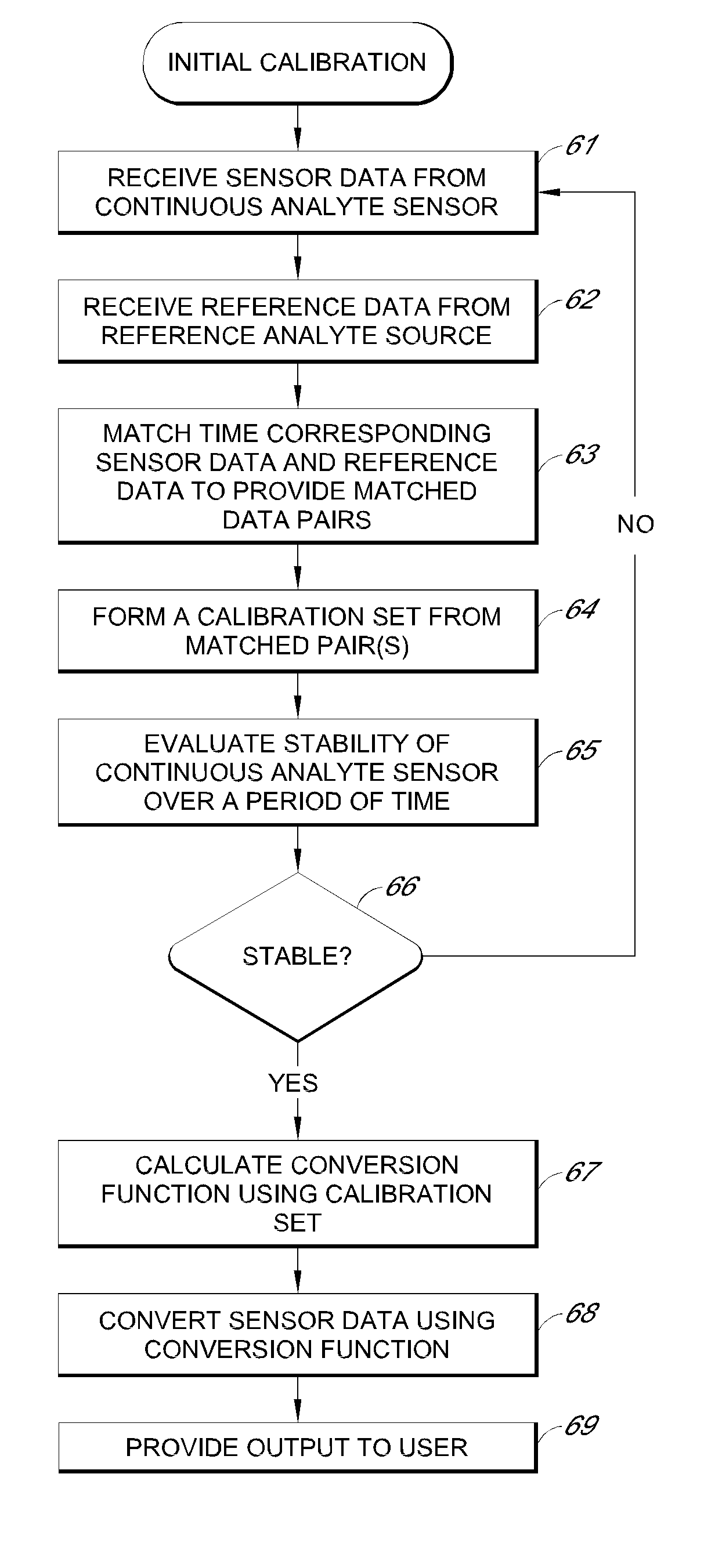
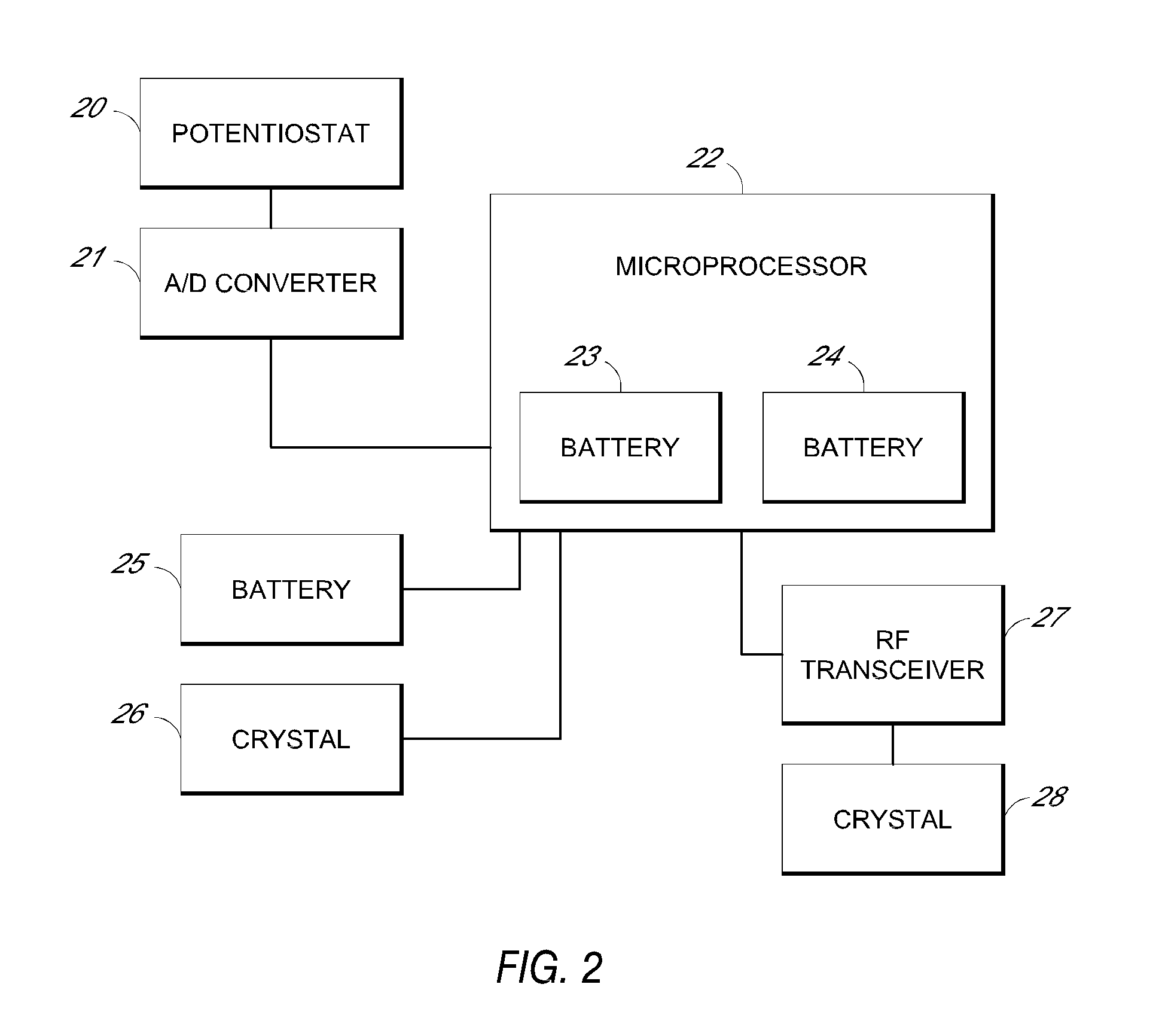
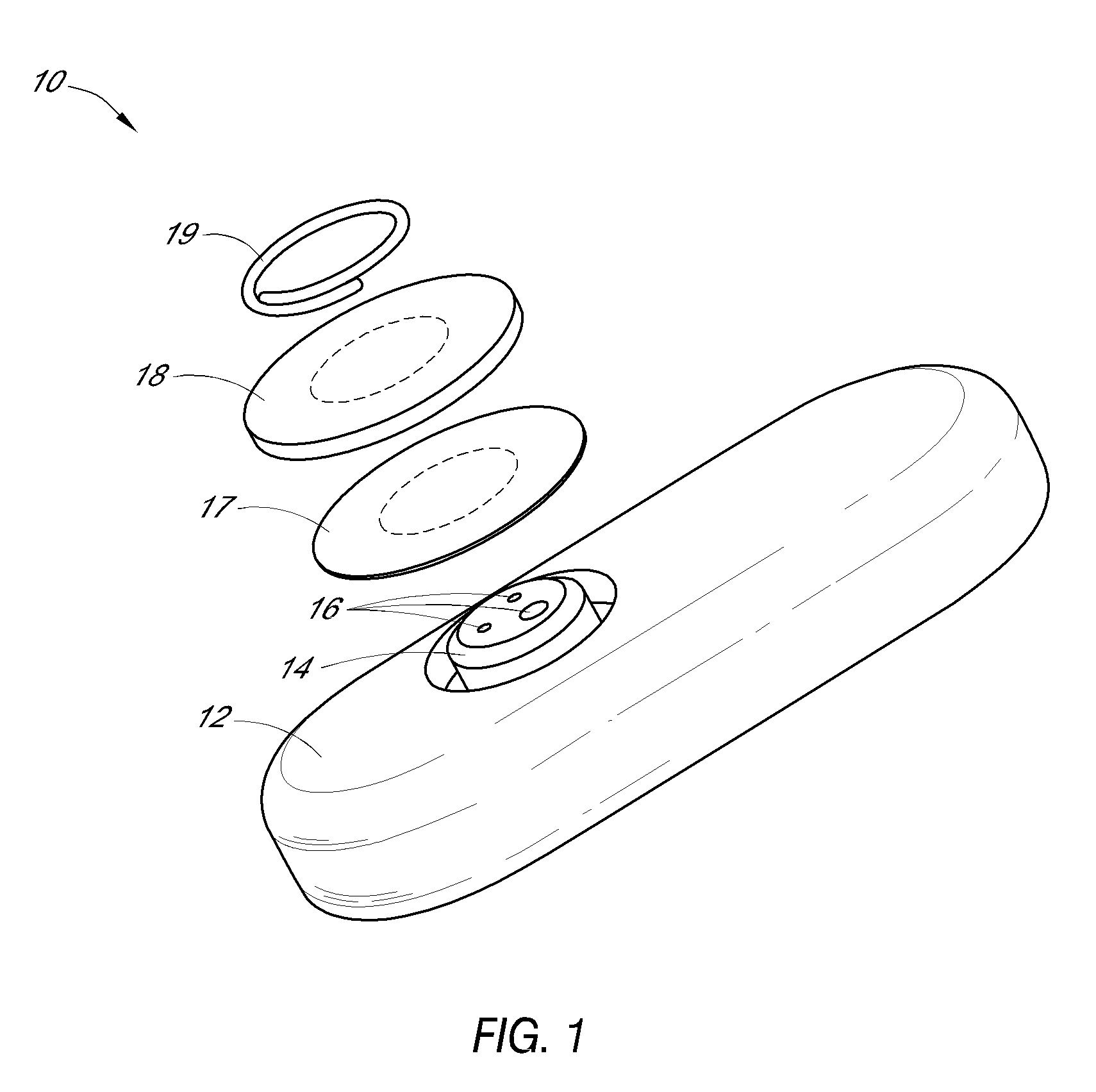
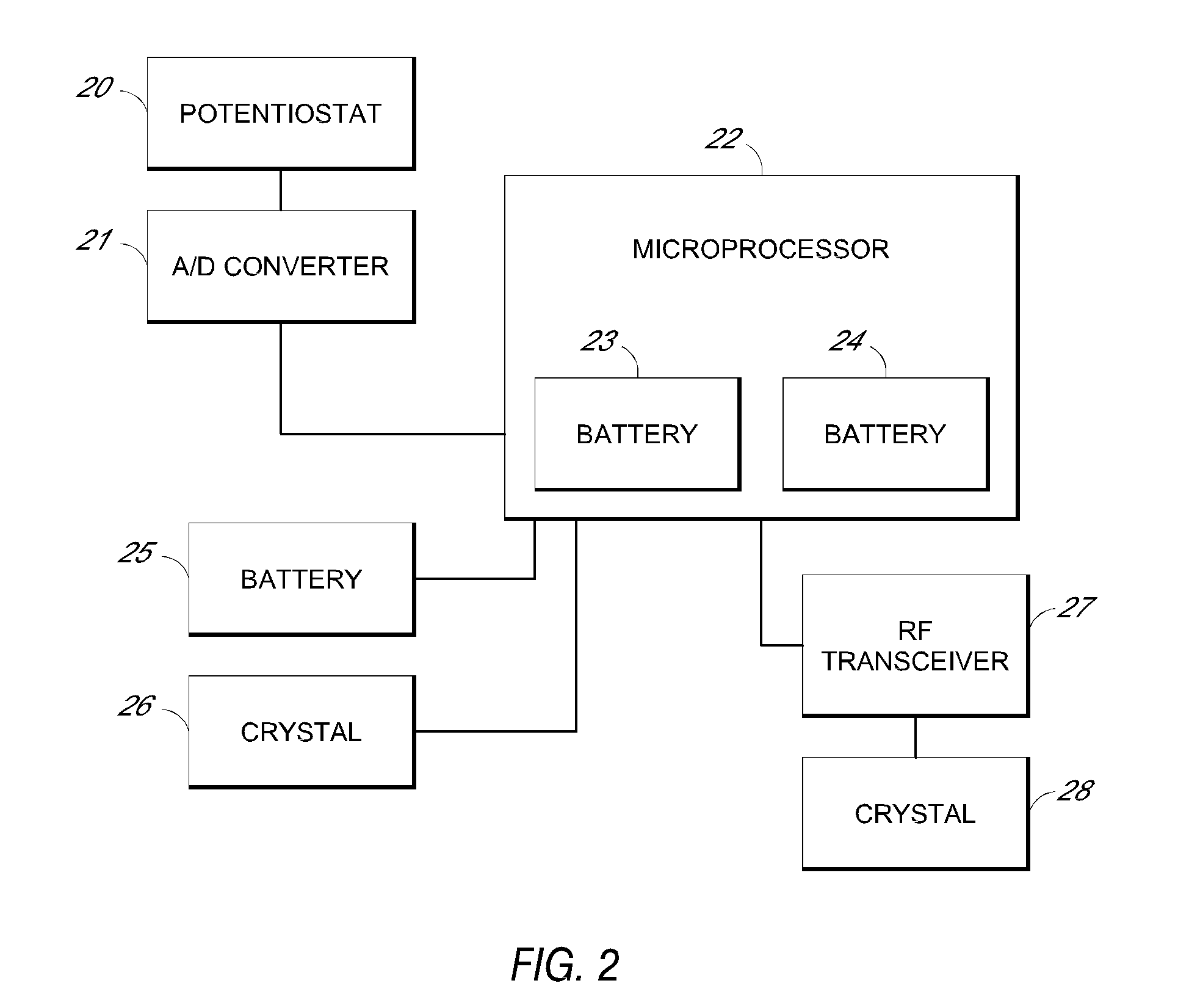
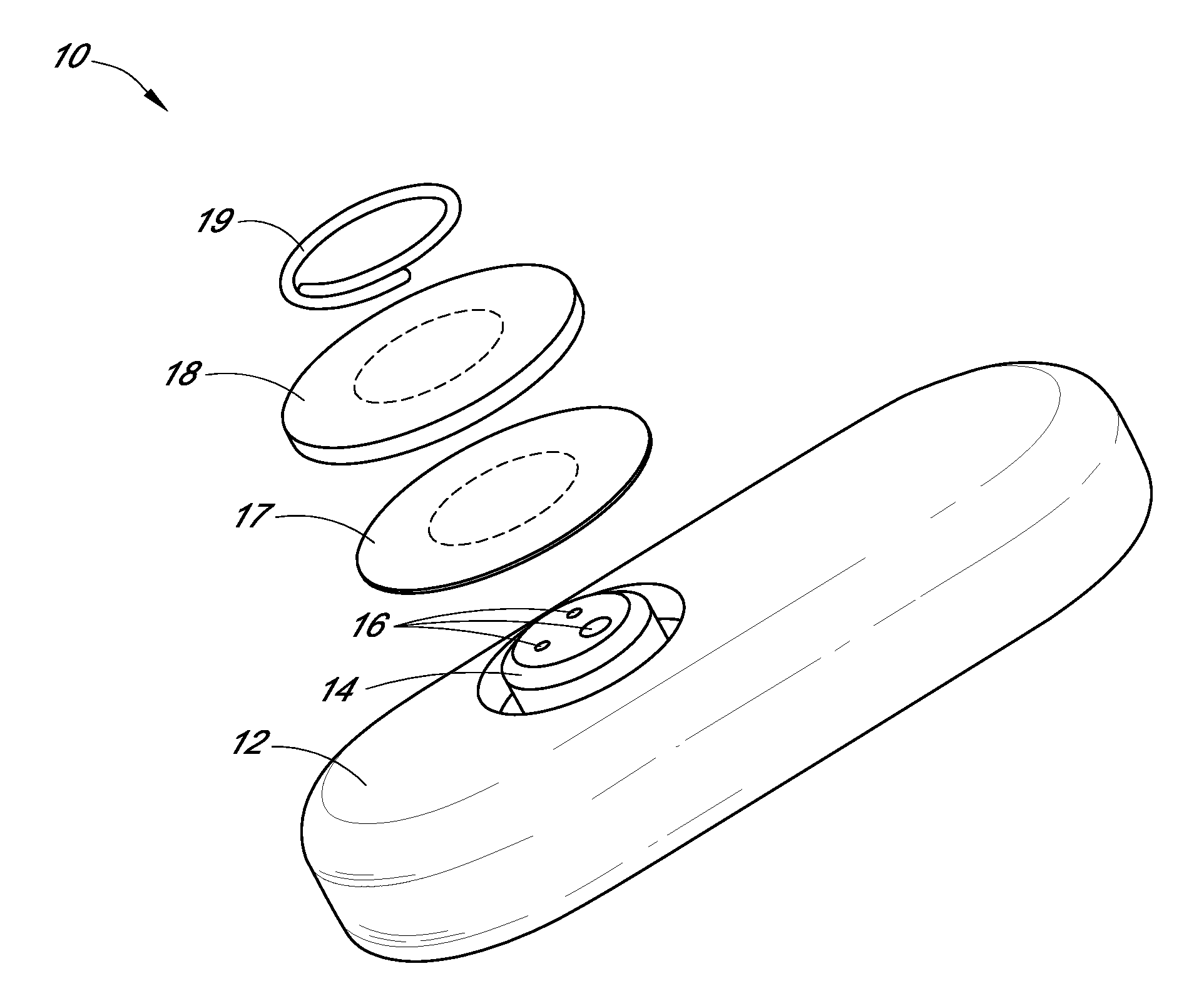
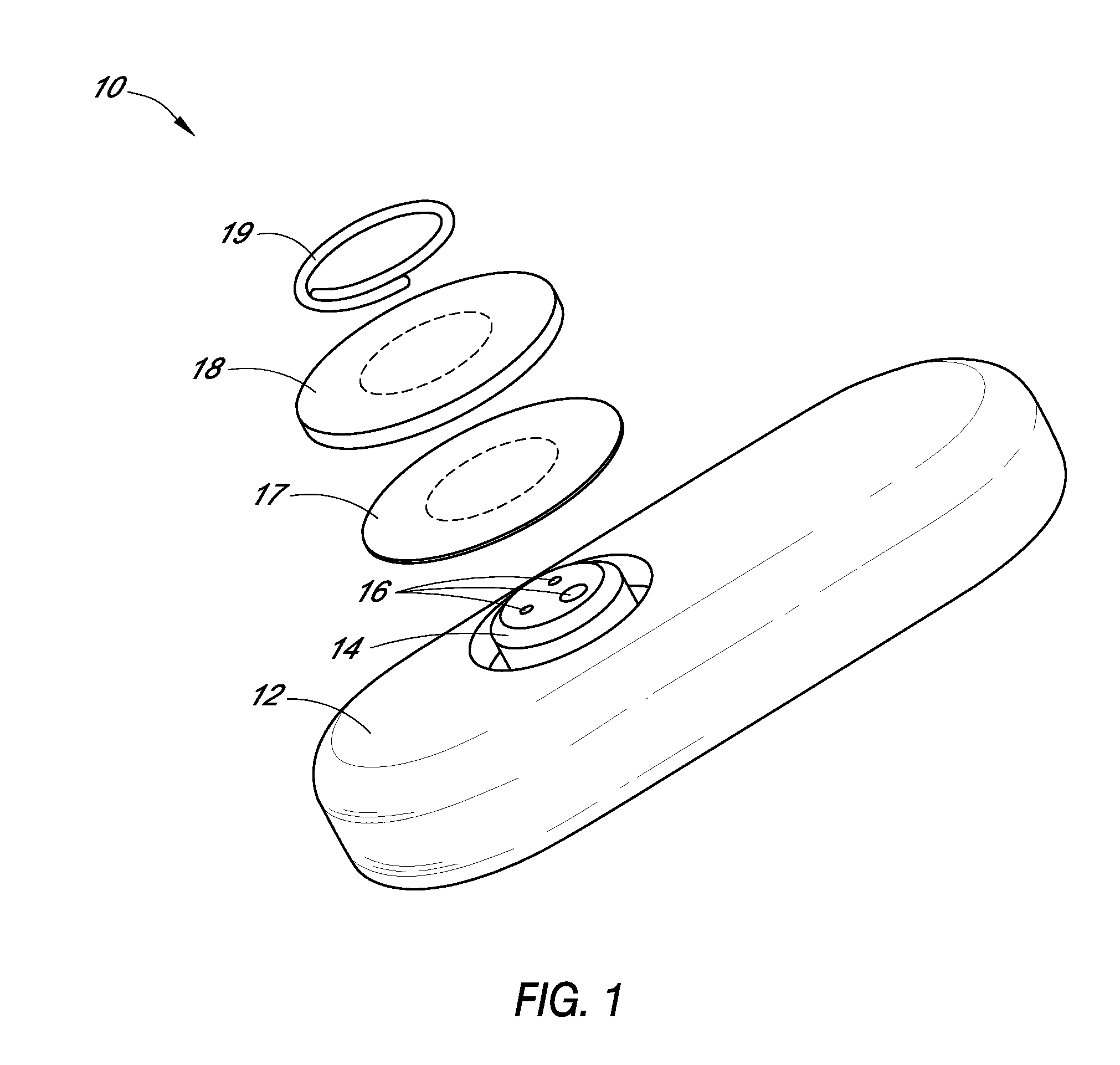
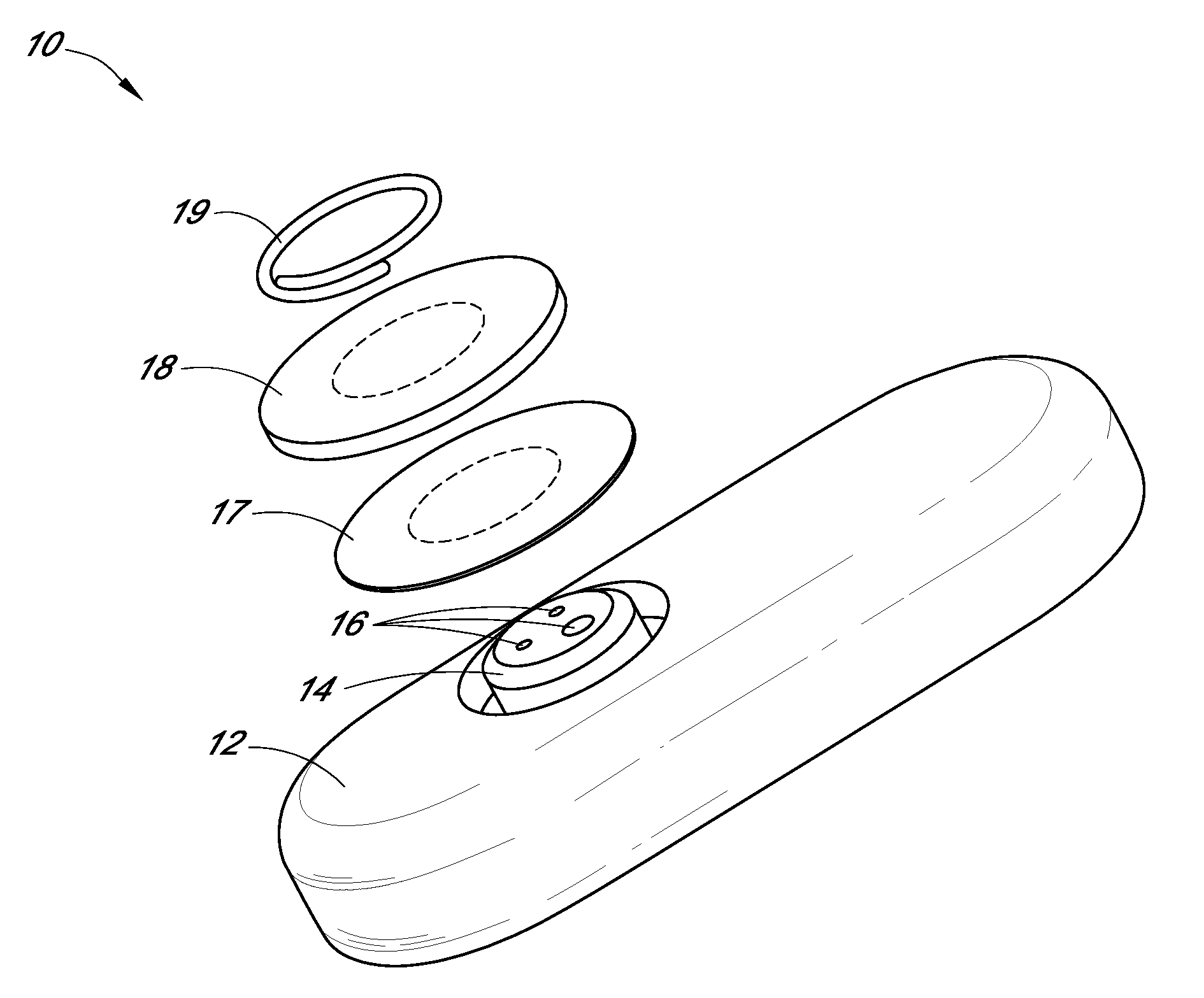
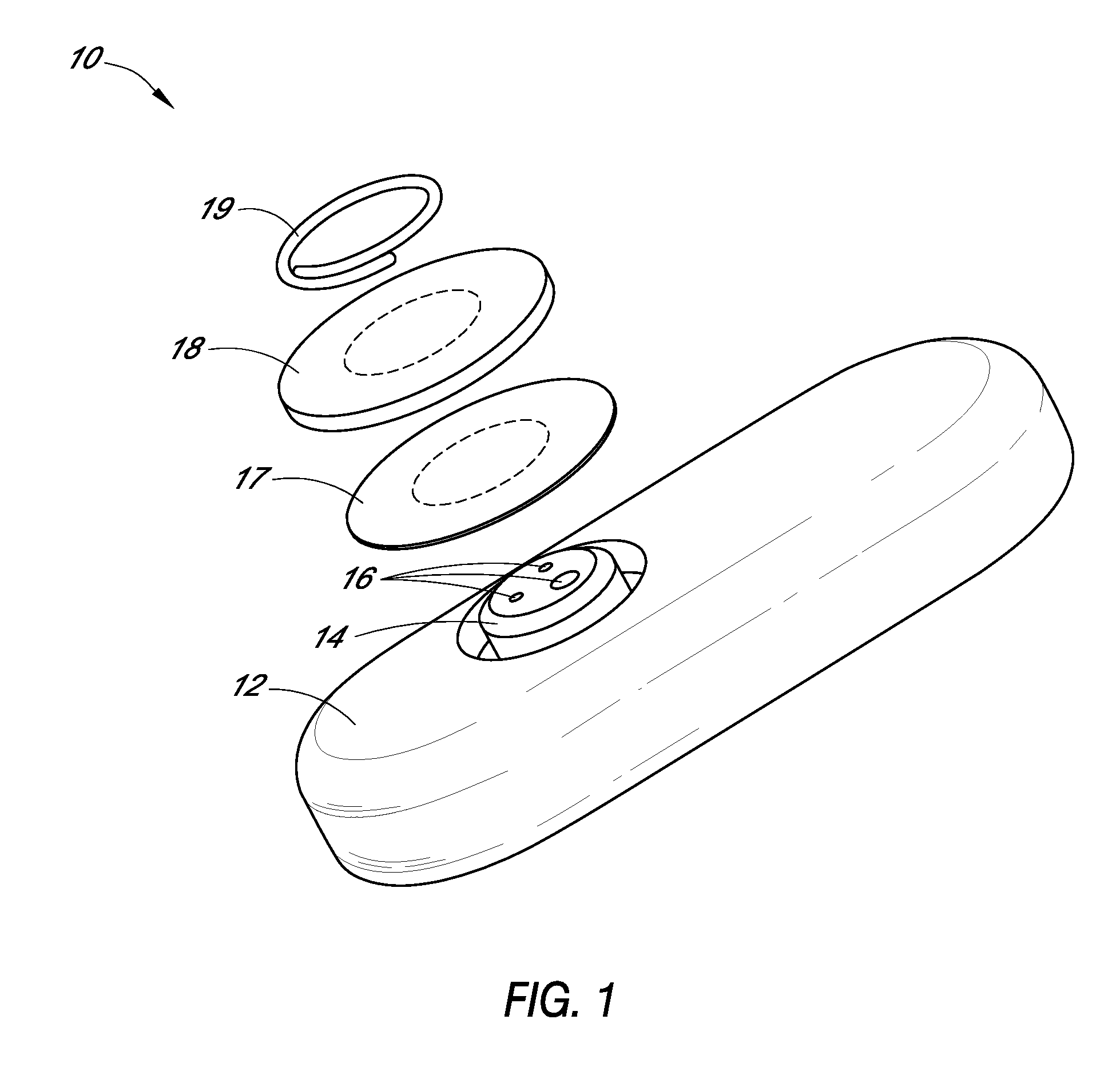
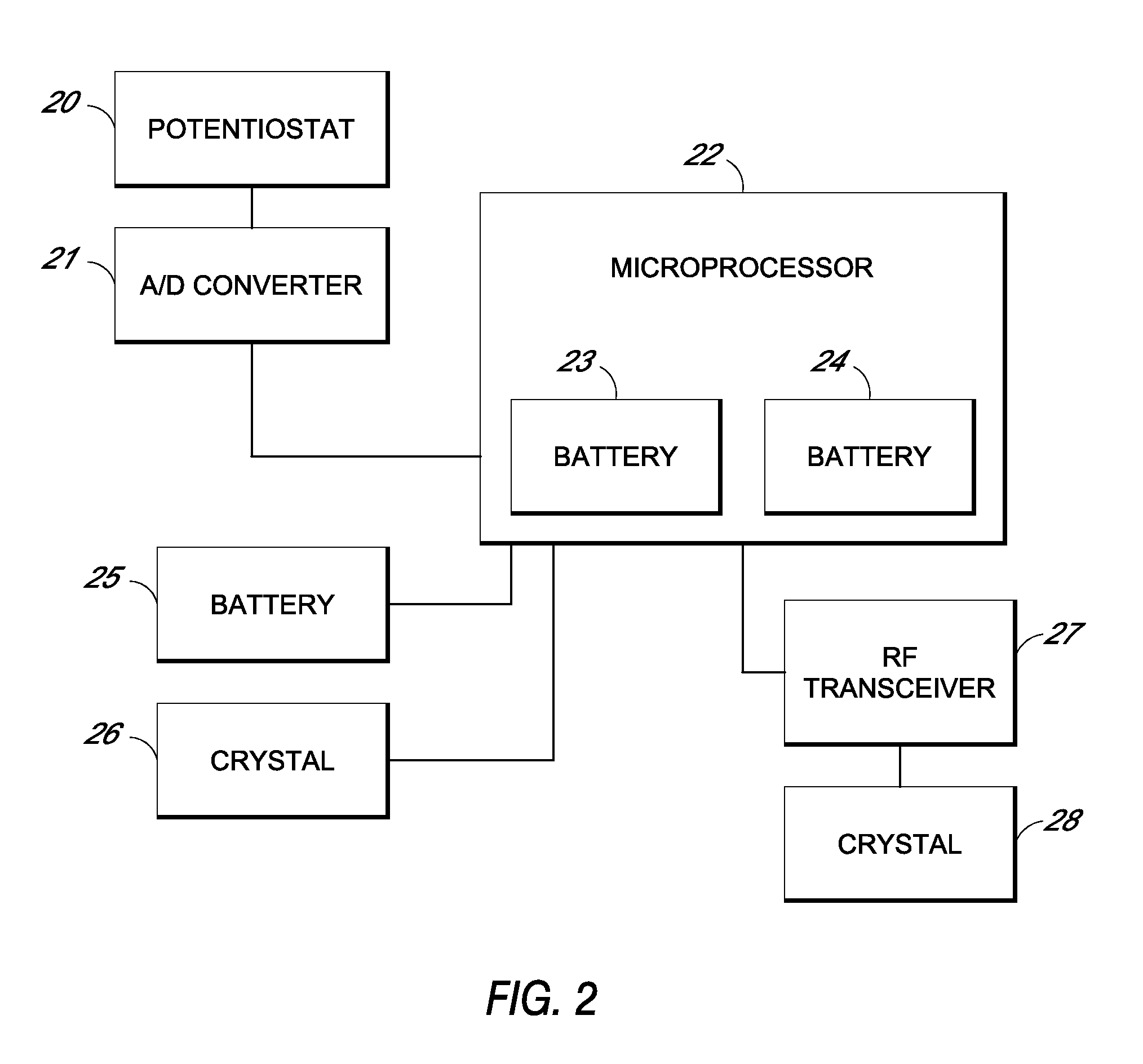
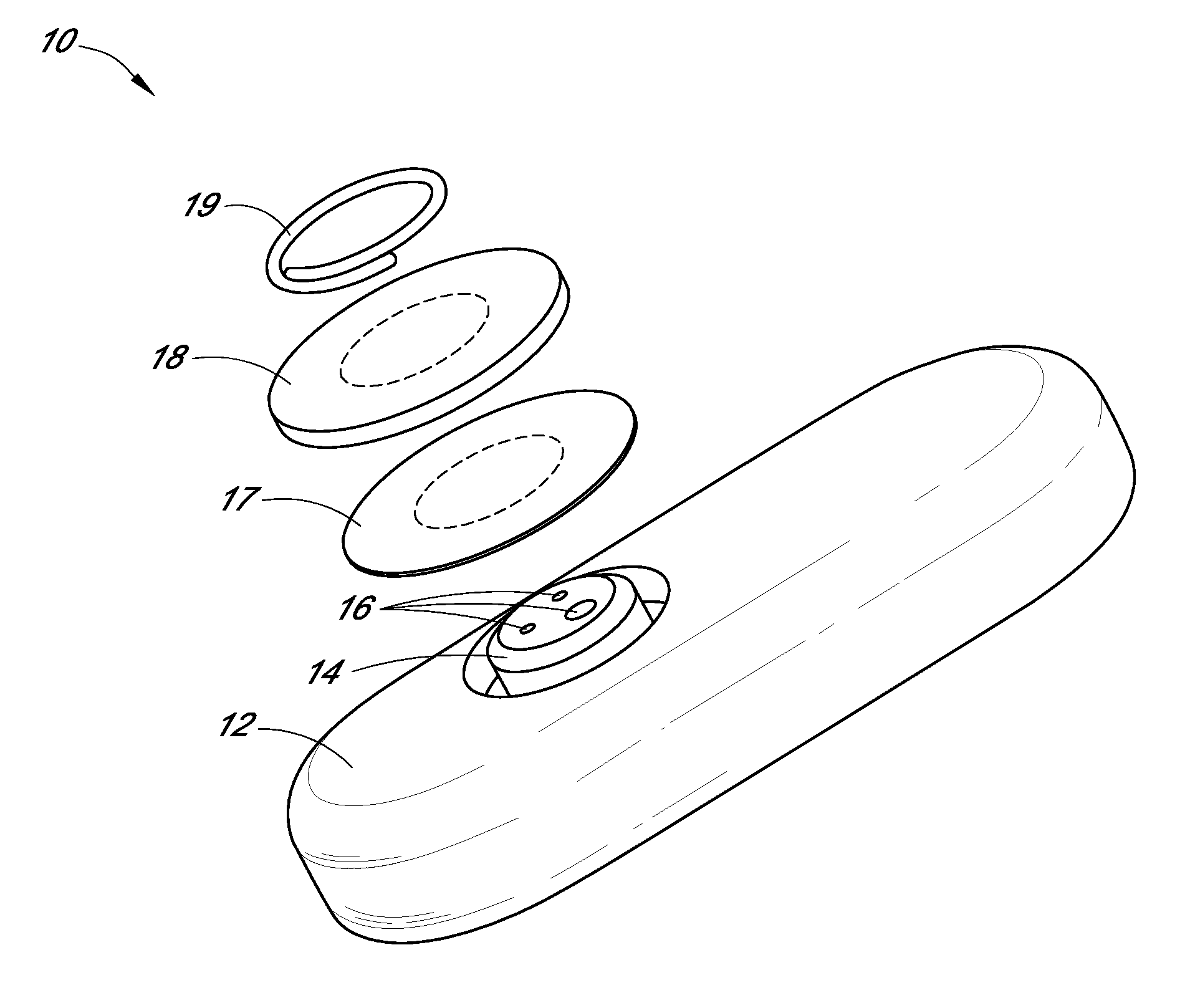
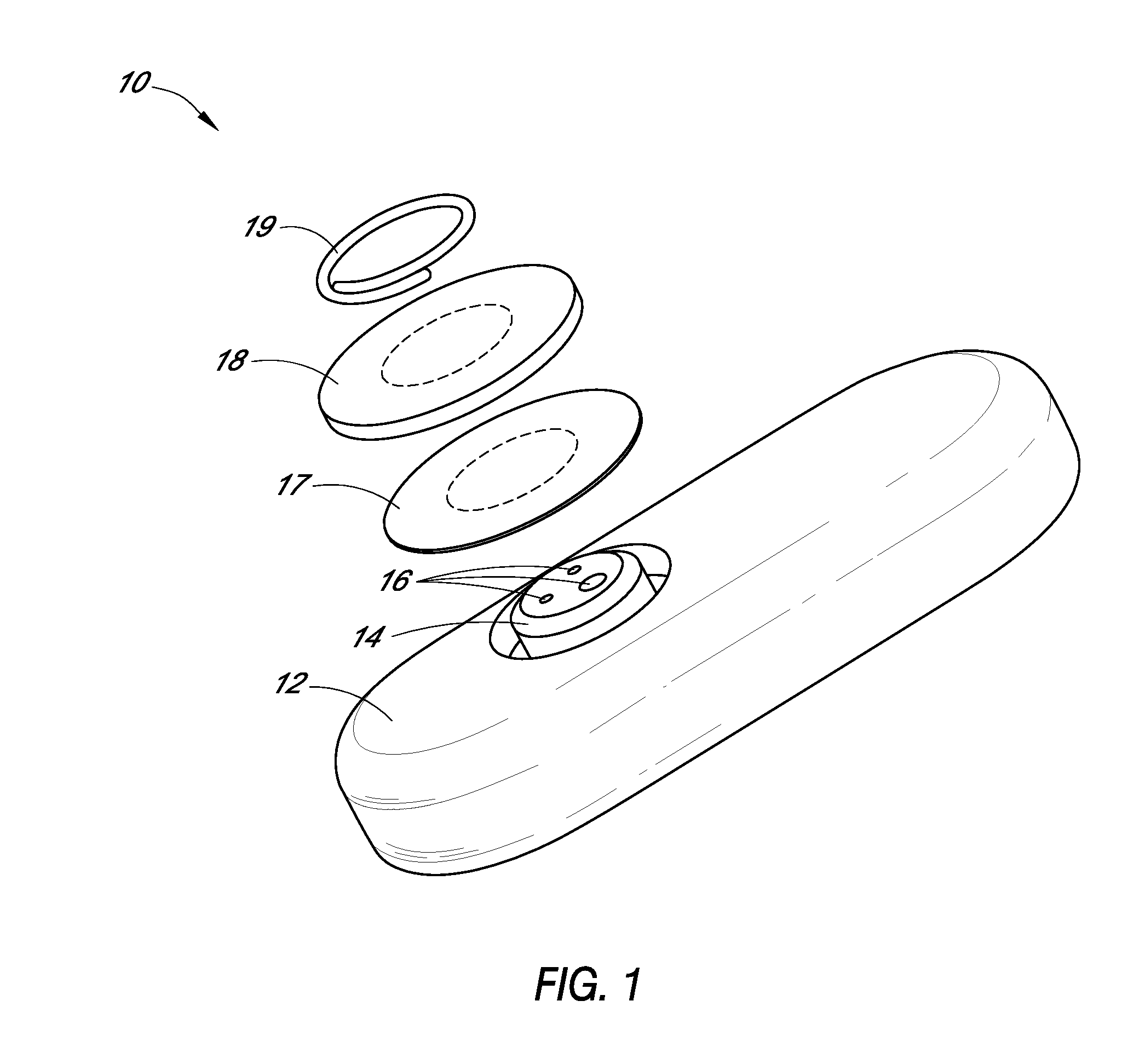
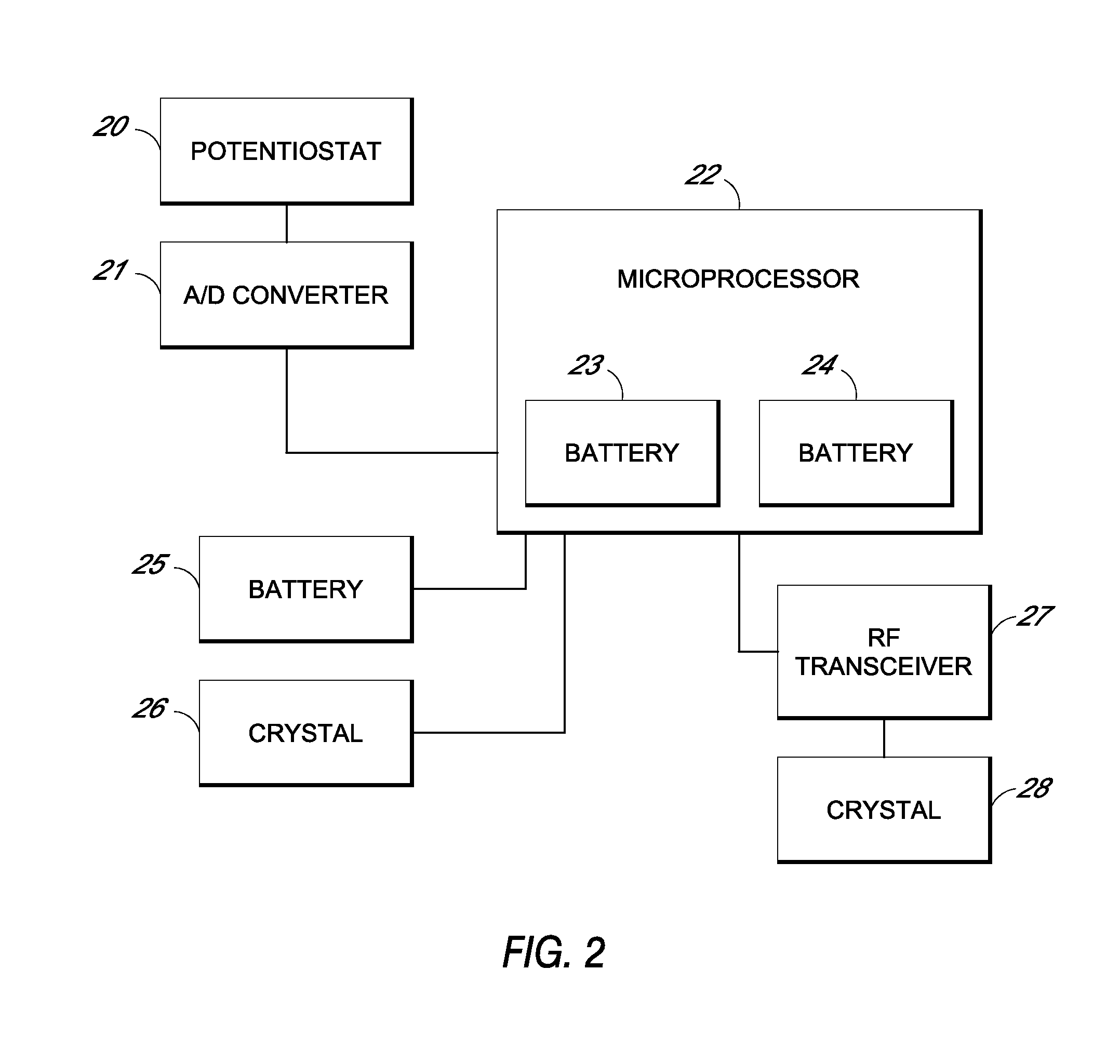
Systems and methods for processing sensor analyte data, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. During initial calibration, the analyte sensor data is evaluated over a period of time to determine stability of the sensor. The sensor may be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. The calibration may be updated after evaluating the calibration set for best calibration based on inclusion criteria with newly received reference analyte data. Fail-safe mechanisms are provided based on clinical acceptability of reference and analyte data and quality of sensor calibration. Algorithms provide for optimized prospective and retrospective analysis of estimated blood analyte data from an analyte sensor.
Owner:DEXCOM
System and methods for processing analyte sensor data
Systems and methods for processing sensor analyte data, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. During initial calibration, the analyte sensor data is evaluated over a period of time to determine stability of the sensor. The sensor may be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. The calibration may be updated after evaluating the calibration set for best calibration based on inclusion criteria with newly received reference analyte data. Fail-safe mechanisms are provided based on clinical acceptability of reference and analyte data and quality of sensor calibration. Algorithms provide for optimized prospective and retrospective analysis of estimated blood analyte data from an analyte sensor.
Owner:DEXCOM
System and methods for processing analyte sensor data
ActiveUS20050027463A1Material thermal conductivityMaterial analysis by electric/magnetic meansAnalyteData system
Systems and methods for processing sensor analyte data, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. During initial calibration, the analyte sensor data is evaluated over a period of time to determine stability of the sensor. The sensor may be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. The calibration may be updated after evaluating the calibration set for best calibration based on inclusion criteria with newly received reference analyte data. Fail-safe mechanisms are provided based on clinical acceptability of reference and analyte data and quality of sensor calibration. Algorithms provide for optimized prospective and retrospective analysis of estimated blood analyte data from an analyte sensor.
Owner:DEXCOM
System and methods for processing analyte sensor data
Systems and methods for processing sensor analyte data, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. During initial calibration, the analyte sensor data is evaluated over a period of time to determine stability of the sensor. The sensor may be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. The calibration may be updated after evaluating the calibration set for best calibration based on inclusion criteria with newly received reference analyte data. Fail-safe mechanisms are provided based on clinical acceptability of reference and analyte data and quality of sensor calibration. Algorithms provide for optimized prospective and retrospective analysis of estimated blood analyte data from an analyte sensor.
Owner:DEXCOM
System and methods for processing analyte sensor data
Systems and methods for processing sensor analyte data, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. During initial calibration, the analyte sensor data is evaluated over a period of time to determine stability of the sensor. The sensor may be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. The calibration may be updated after evaluating the calibration set for best calibration based on inclusion criteria with newly received reference analyte data. Fail-safe mechanisms are provided based on clinical acceptability of reference and analyte data and quality of sensor calibration. Algorithms provide for optimized prospective and retrospective analysis of estimated blood analyte data from an analyte sensor.
Owner:DEXCOM
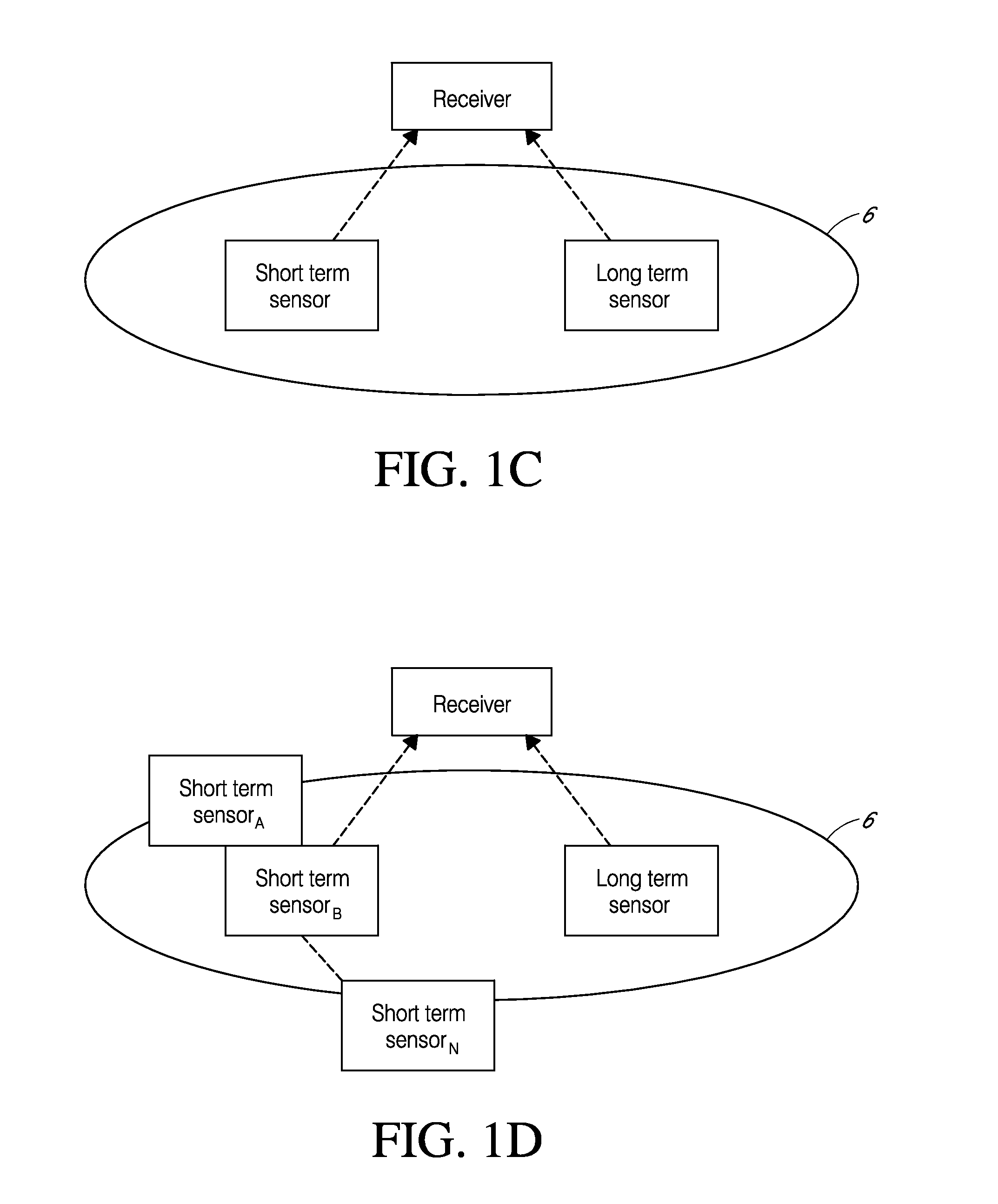
System and methods for processing analyte sensor data for sensor calibration
Systems and methods for processing sensor analyte data are disclosed, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. The sensor can be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. Reference data resulting from benchtop testing an analyte sensor prior to its insertion can be used to provide initial calibration of the sensor data. Reference data from a short term continuous analyte sensor implanted in a user can be used to initially calibrate or update sensor data from a long term continuous analyte sensor.
Owner:DEXCOM INC
Apparatus and method for non-invasive spectroscopic measurement of analytes in tissue using a matched reference analyte
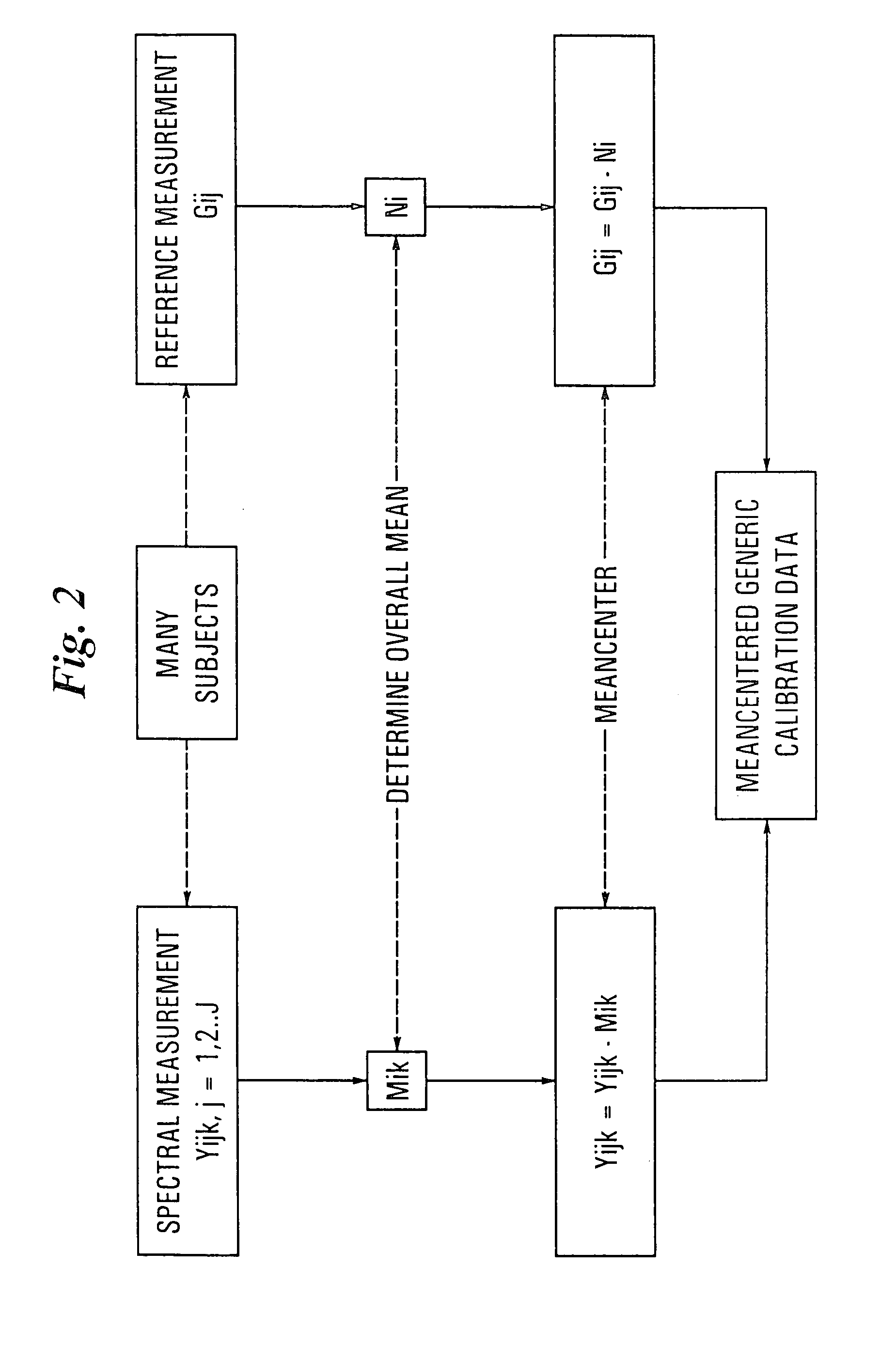
A method is provided for building improved calibration models or for improving modifications to these models or validation of the models used in the non-invasive spectroscopic measurement of an analyte or attribute of tissue. The method uses a matched reference sample and measurement of that sample to ensure that the correct relationship between the spectra and analyte is made during the model building, modification or calibration process. A matched reference sample is one in which the analyte of interest or attribute of interest in the reference sample is representative of the analyte or attribute at the site being non-invasively sampled or the agreement between the reference concentration and the non-invasively sampled concentration is clinically significant.
Owner:NEW MEXICO UNIV OF +1
System and methods for processing analyte sensor data
InactiveUS20110231140A1Testing/calibration apparatusSpeed measurement using gyroscopic effectsAnalyteData system
Owner:DEXCOM INC
System and methods for processing analyte sensor data
ActiveUS20110231142A1Testing/calibration apparatusSpeed measurement using gyroscopic effectsAnalyteData system
Owner:DEXCOM INC
System and methods for processing analyte sensor data
Systems and methods for processing sensor analyte data, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. During initial calibration, the analyte sensor data is evaluated over a period of time to determine stability of the sensor. The sensor may be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. The calibration may be updated after evaluating the calibration set for best calibration based on inclusion criteria with newly received reference analyte data. Fail-safe mechanisms are provided based on clinical acceptability of reference and analyte data and quality of sensor calibration. Algorithms provide for optimized prospective and retrospective analysis of estimated blood analyte data from an analyte sensor.
Owner:DEXCOM
System and methods for processing analyte sensor data for sensor calibration
Systems and methods for processing sensor analyte data are disclosed, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. The sensor can be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. Reference data resulting from benchtop testing an analyte sensor prior to its insertion can be used to provide initial calibration of the sensor data. Reference data from a short term continuous analyte sensor implanted in a user can be used to initially calibrate or update sensor data from a long term continuous analyte sensor.
Owner:DEXCOM INC
System and methods for processing analyte sensor data
Systems and methods for processing sensor analyte data, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. During initial calibration, the analyte sensor data is evaluated over a period of time to determine stability of the sensor. The sensor may be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. The calibration may be updated after evaluating the calibration set for best calibration based on inclusion criteria with newly received reference analyte data. Fail-safe mechanisms are provided based on clinical acceptability of reference and analyte data and quality of sensor calibration. Algorithms provide for optimized prospective and retrospective analysis of estimated blood analyte data from an analyte sensor.
Owner:DEXCOM
System and methods for processing analyte sensor data
Systems and methods for processing sensor analyte data, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. During initial calibration, the analyte sensor data is evaluated over a period of time to determine stability of the sensor. The sensor may be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. The calibration may be updated after evaluating the calibration set for best calibration based on inclusion criteria with newly received reference analyte data. Fail-safe mechanisms are provided based on clinical acceptability of reference and analyte data and quality of sensor calibration. Algorithms provide for optimized prospective and retrospective analysis of estimated blood analyte data from an analyte sensor.
Owner:DEXCOM
System and methods for processing analyte sensor data
Systems and methods for processing sensor analyte data, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. During initial calibration, the analyte sensor data is evaluated over a period of time to determine stability of the sensor. The sensor may be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. The calibration may be updated after evaluating the calibration set for best calibration based on inclusion criteria with newly received reference analyte data. Fail-safe mechanisms are provided based on clinical acceptability of reference and analyte data and quality of sensor calibration. Algorithms provide for optimized prospective and retrospective analysis of estimated blood analyte data from an analyte sensor.
Owner:DEXCOM
System and methods for processing analyte sensor data
Systems and methods for processing sensor analyte data, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. During initial calibration, the analyte sensor data is evaluated over a period of time to determine stability of the sensor. The sensor may be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. The calibration may be updated after evaluating the calibration set for best calibration based on inclusion criteria with newly received reference analyte data. Fail-safe mechanisms are provided based on clinical acceptability of reference and analyte data and quality of sensor calibration. Algorithms provide for optimized prospective and retrospective analysis of estimated blood analyte data from an analyte sensor.
Owner:DEXCOM
System and methods for processing analyte sensor data
Systems and methods for processing sensor analyte data, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. During initial calibration, the analyte sensor data is evaluated over a period of time to determine stability of the sensor. The sensor may be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. The calibration may be updated after evaluating the calibration set for best calibration based on inclusion criteria with newly received reference analyte data. Fail-safe mechanisms are provided based on clinical acceptability of reference and analyte data and quality of sensor calibration. Algorithms provide for optimized prospective and retrospective analysis of estimated blood analyte data from an analyte sensor.
Owner:DEXCOM INC
System and methods for processing analyte sensor data
Systems and methods for processing sensor analyte data, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. During initial calibration, the analyte sensor data is evaluated over a period of time to determine stability of the sensor. The sensor may be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. The calibration may be updated after evaluating the calibration set for best calibration based on inclusion criteria with newly received reference analyte data. Fail-safe mechanisms are provided based on clinical acceptability of reference and analyte data and quality of sensor calibration. Algorithms provide for optimized prospective and retrospective analysis of estimated blood analyte data from an analyte sensor.
Owner:DEXCOM
Method of sample control and calibration adjustment for use with a noninvasive analyzer
InactiveUS20050014997A1Reduce variationMaintain abilityMaterial analysis by optical meansDiagnostic recording/measuringFrequency spectrumAnalyte
A method and apparatus for easing the use of an optically based noninvasive analyzer is presented. More particularly, a simplified algorithm is used that removes the daily requirement of collecting and using a noninvasive spectrum to update a calibration model. In another embodiment, a guide is used to substantially reduce variation in sample probe placement in relation to a skin tissue sampling site, resulting in the ability to maintain calibration performance with the use of a reference analyte concentration, with or without the use of a reference spectrum collected nearby in time.
Owner:GLT ACQUISITION
System and methods for processing analyte sensor data
Systems and methods for processing sensor analyte data, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. During initial calibration, the analyte sensor data is evaluated over a period of time to determine stability of the sensor. The sensor may be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. The calibration may be updated after evaluating the calibration set for best calibration based on inclusion criteria with newly received reference analyte data. Fail-safe mechanisms are provided based on clinical acceptability of reference and analyte data and quality of sensor calibration. Algorithms provide for optimized prospective and retrospective analysis of estimated blood analyte data from an analyte sensor.
Owner:DEXCOM INC
Method and kit for the transdermal determination of analyte concentration in blood
InactiveUS7004901B2Efficient extractionTesting is superfluousDiagnostic recording/measuringSensorsAnalyteMedicine
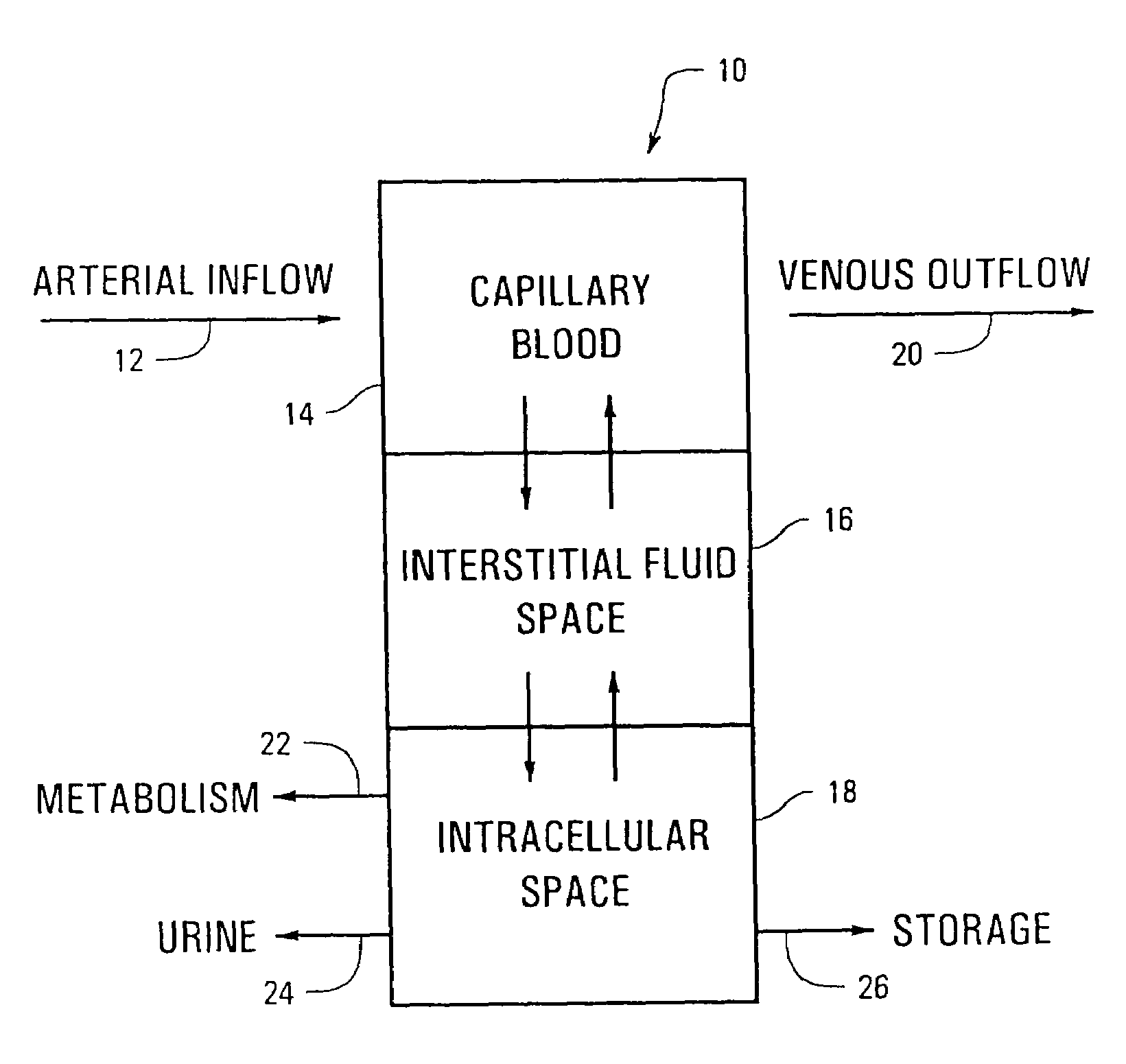
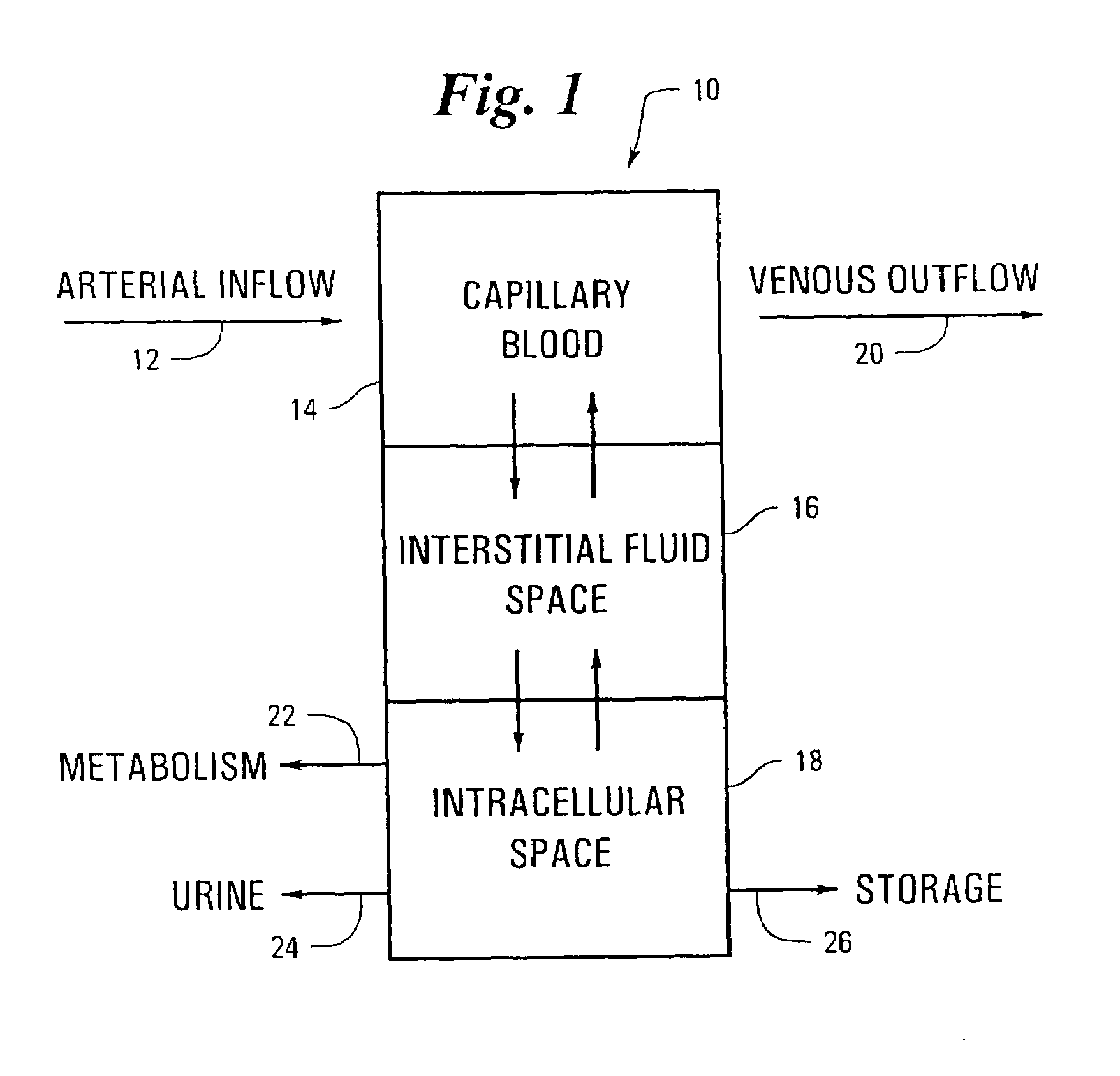
A method is provided for determining the level of an analyte in the blood of an individual by measuring the level of the analyte in an interstitial fluid or in any other non blood fluid which does not contain red blood cells and adjusting the measurement value by the concentration of at least one reference analyte.
Owner:FISH FALK
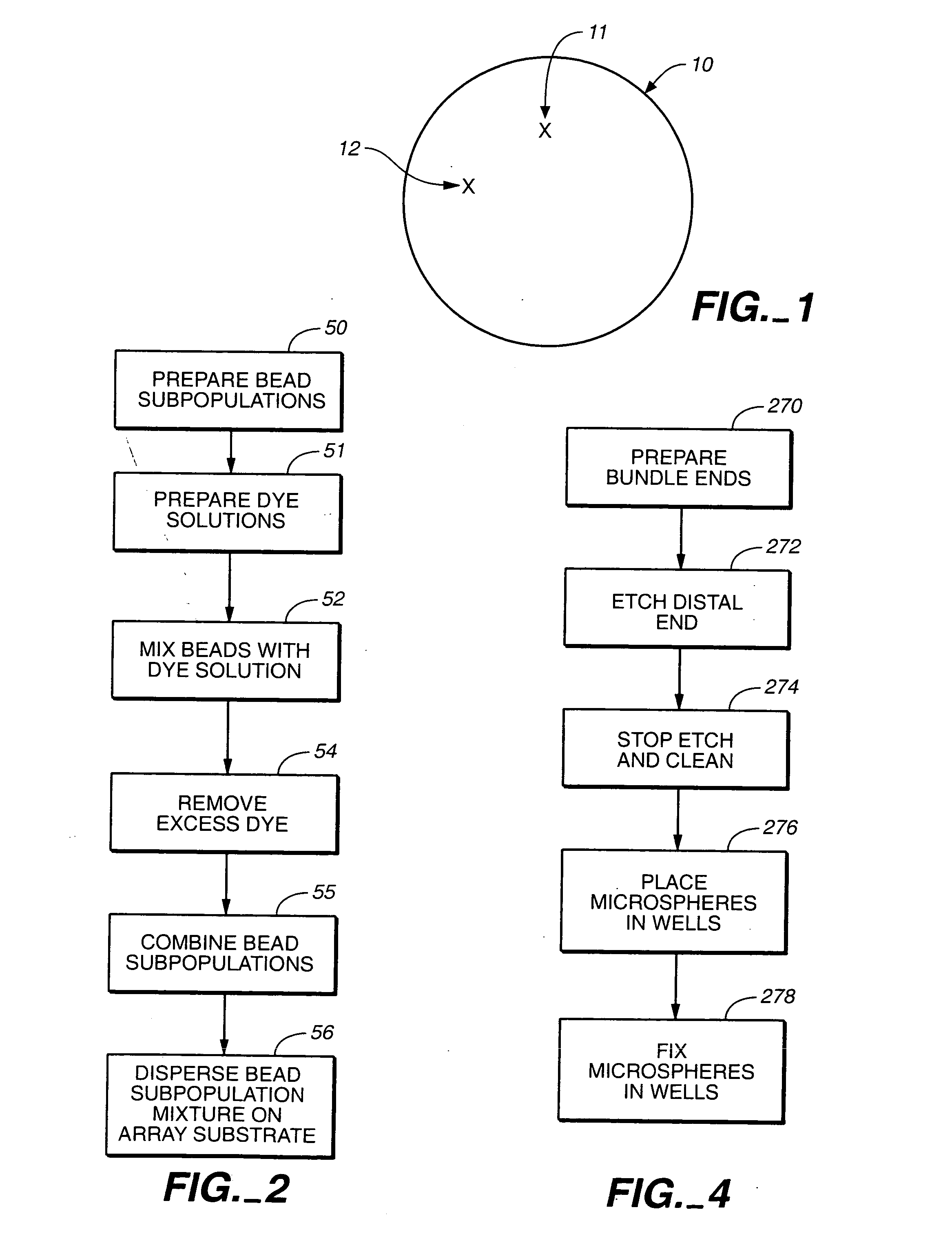
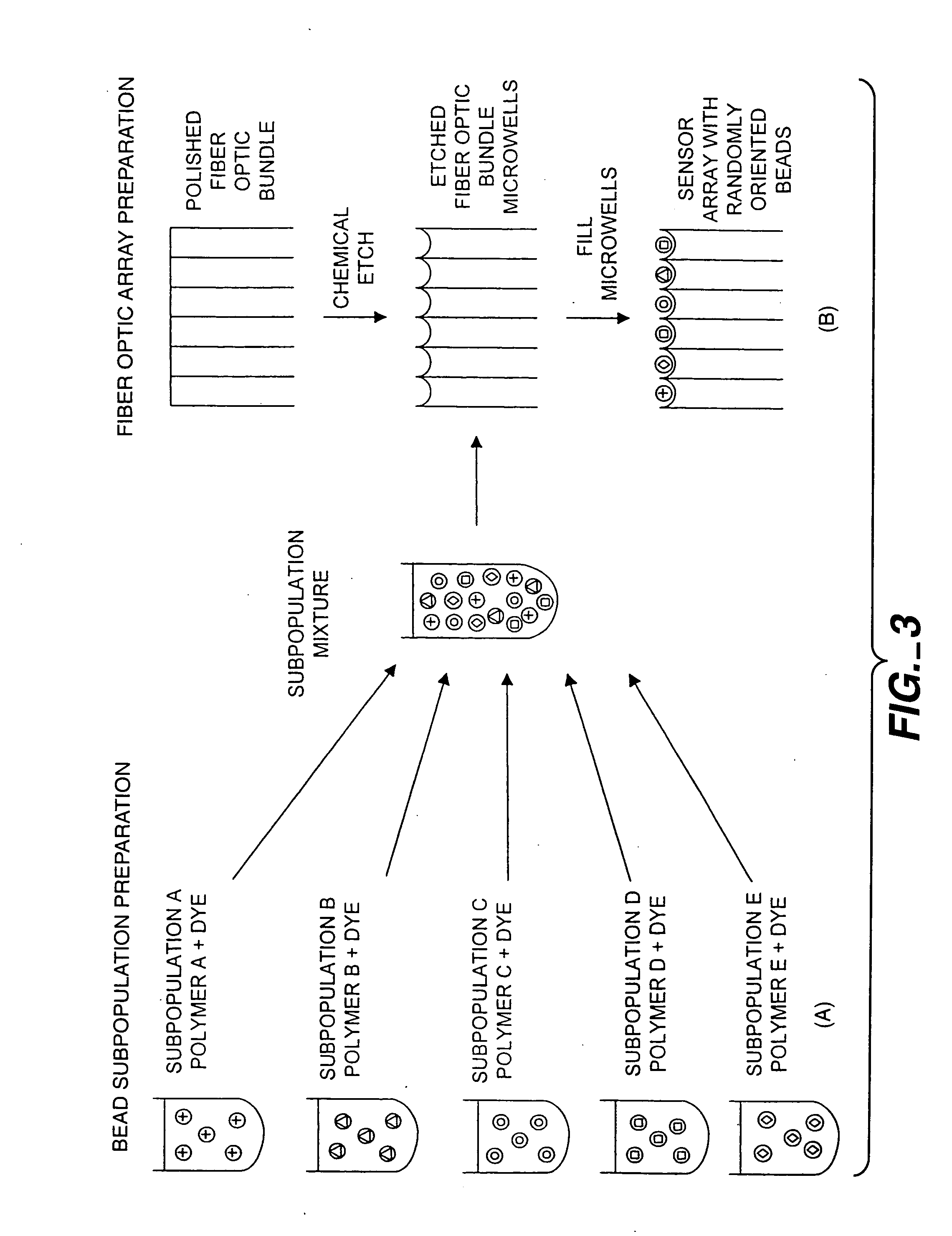
Self-encoding sensor with microspheres
InactiveUS7348181B2Bioreactor/fermenter combinationsBiological substance pretreatmentsSensor arrayMicrosphere
Owner:TRUSTEES OF TUFTS COLLEGE
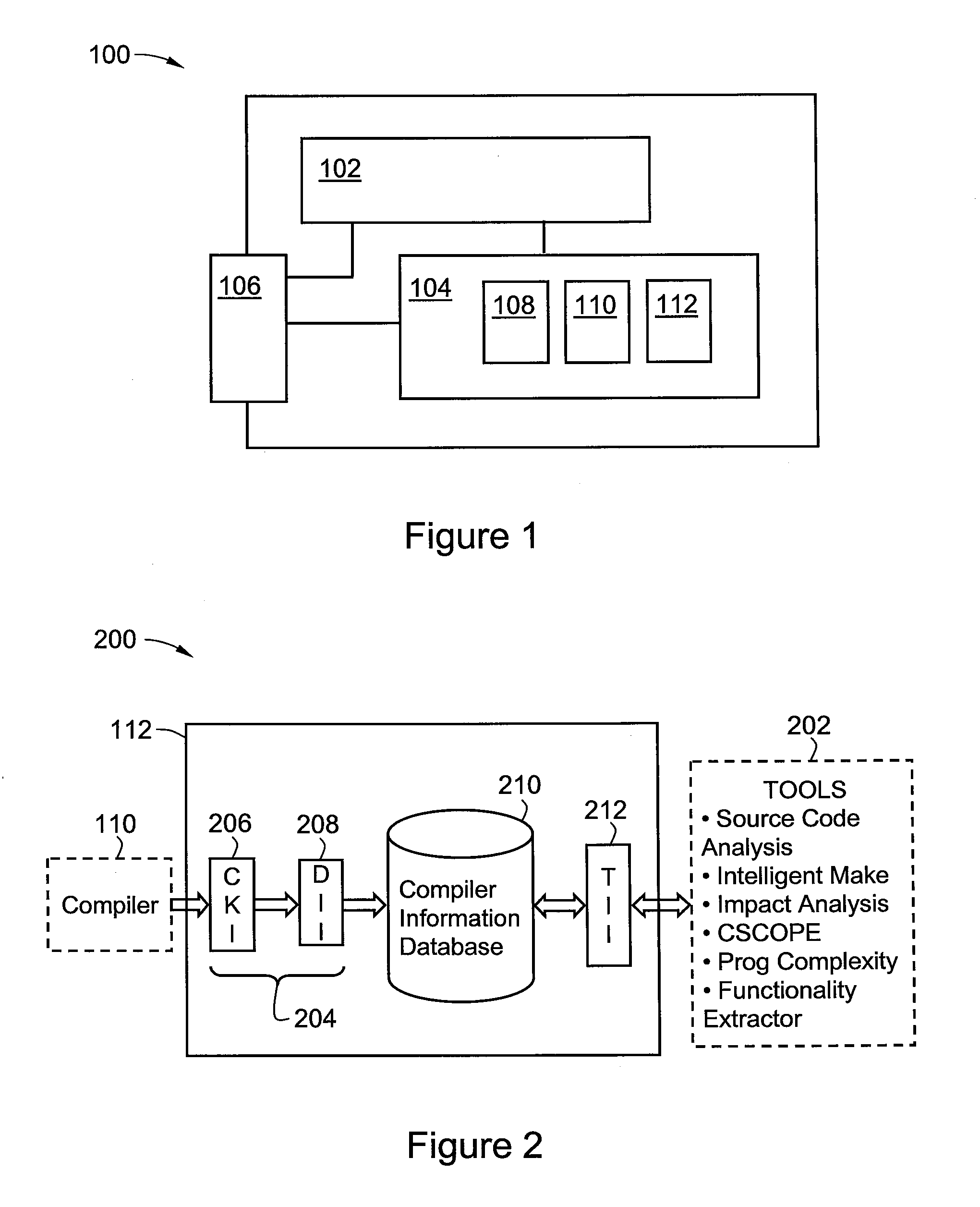
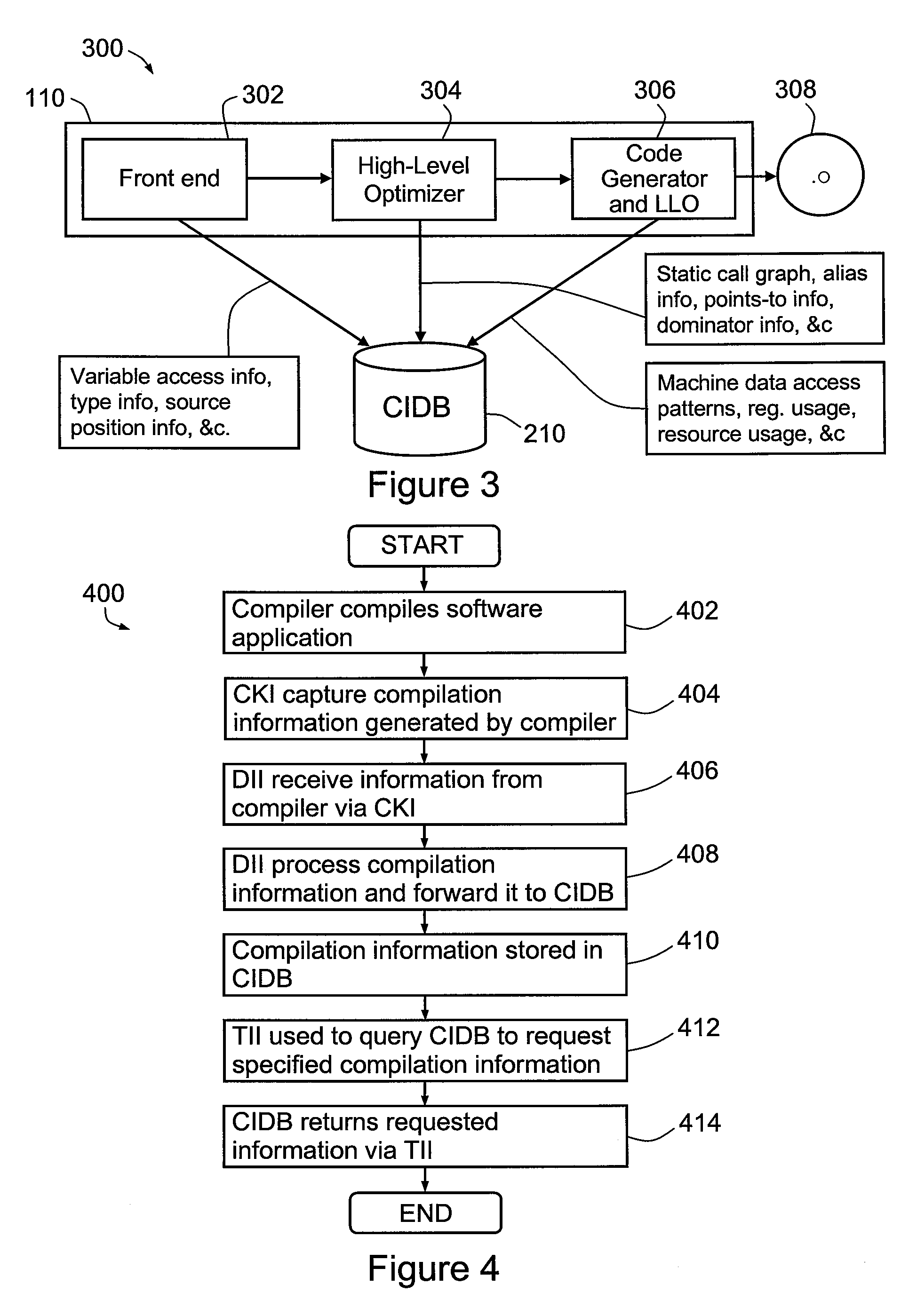
Software development system
InactiveUS20080104096A1Digital data processing detailsRequirement analysisSoftware development processAgile software development
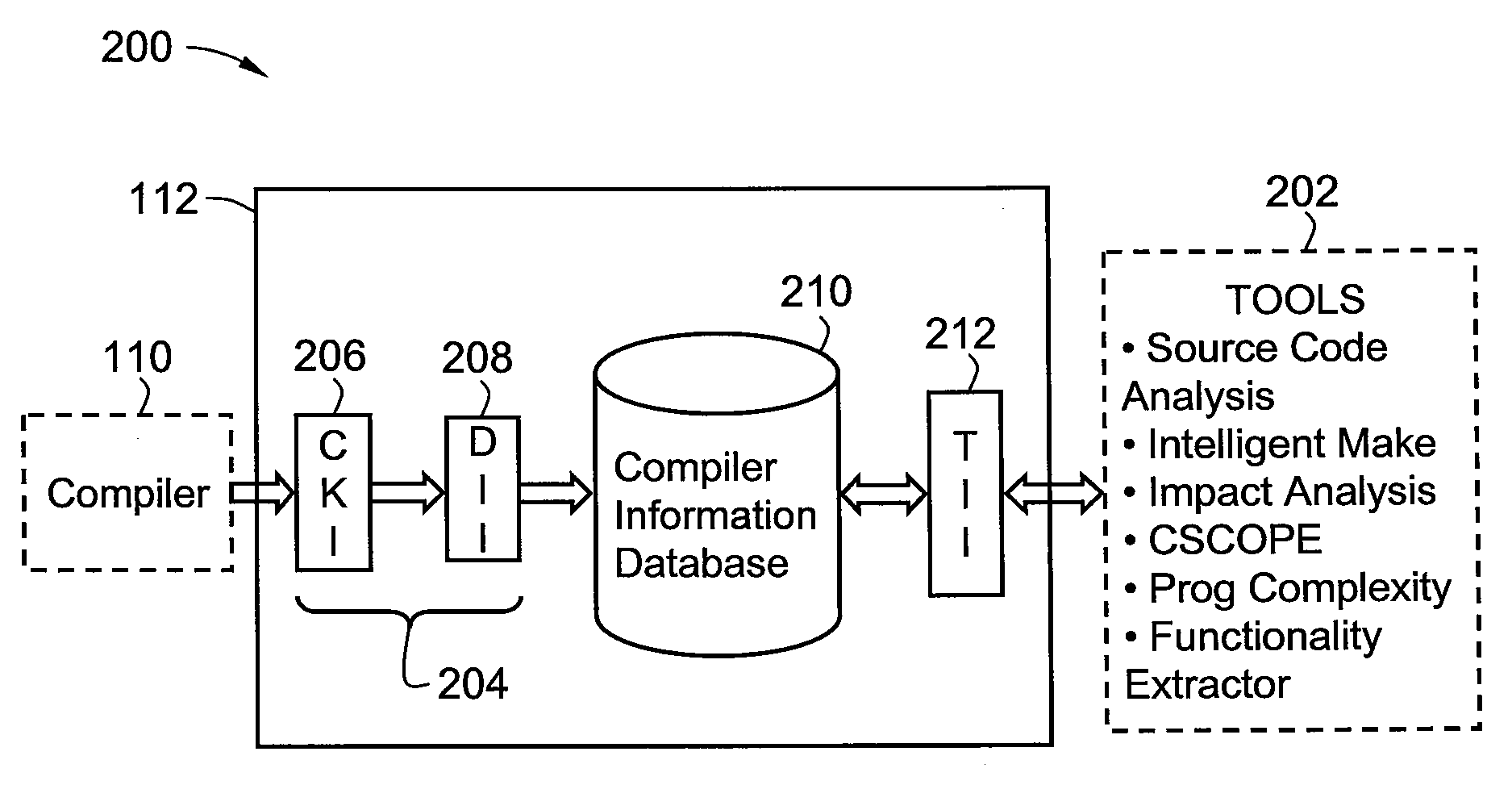
A method and system for developing a software tool. The method comprises capturing analysis information generated by at least one software product and required for or useful in developing the software tool, forwarding the analysis information from the software product to a database, storing the analysis information in the database, querying the database for at least a portion of the analysis information, receiving the portion of the analysis information from the database in response to the querying, and developing the software tool with or by reference to the portion of the analysis information.
Owner:HEWLETT PACKARD DEV CO LP
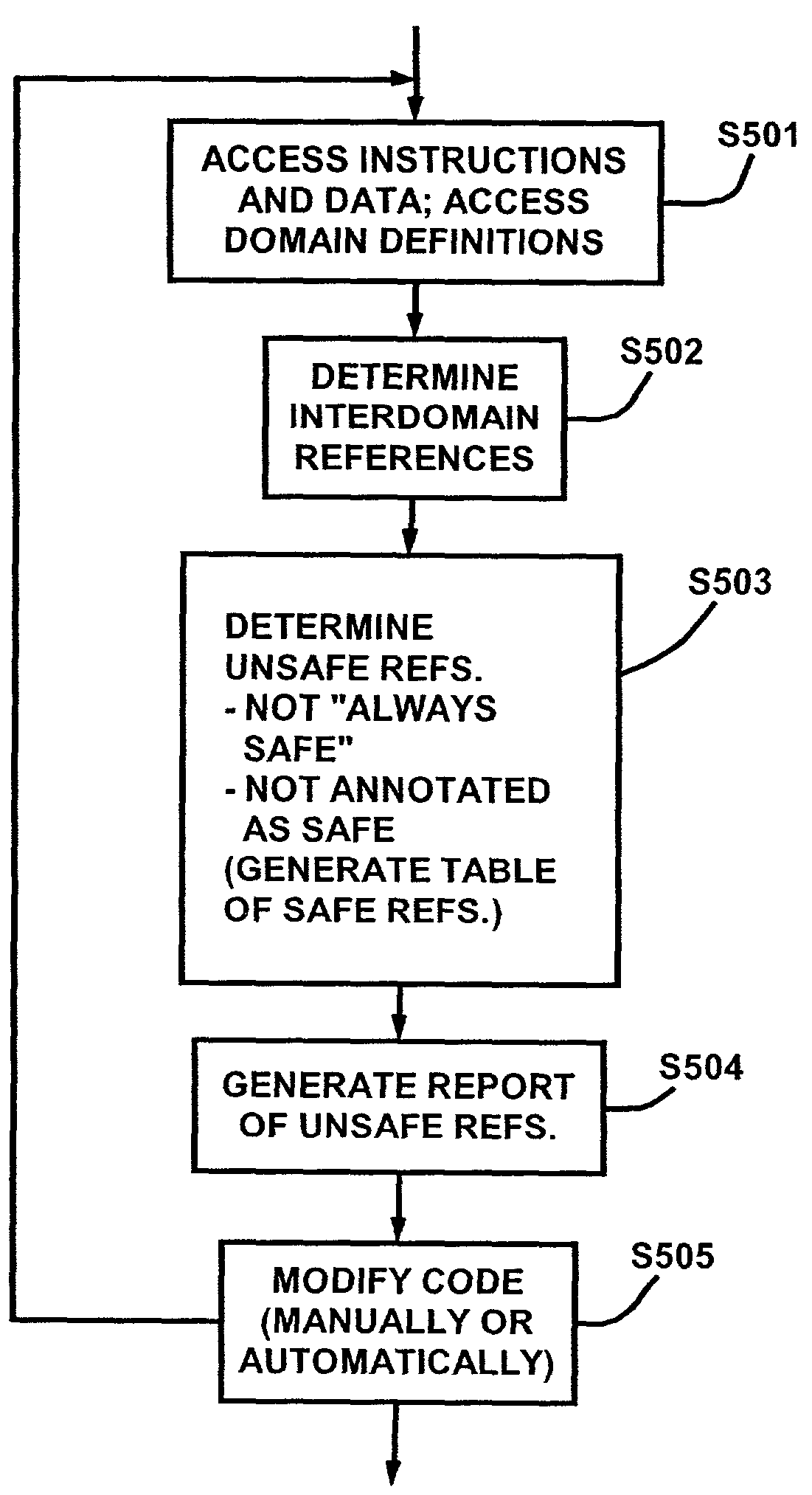
Automatic verification of scheduling domain consistency
InactiveUS7178137B1Specific program execution arrangementsMemory systemsMulti processorParallel computing
An analyzer that analyzes instructions and data to determine where the instructions and data might result in incorrect results when run on a multiprocessor system. The instructions and data are divided into plural domains based on the symbols used to refer to those instructions and data, and the multiprocessor system is configured to use at most one processor at a time to execute instructions and to access data from any one domain. The analyzer preferably includes a reference analyzer and a table generator. The reference analyzer determines which of the instructions and data involve references outside of their domains, and determines which of the references outside of their domains are multiprocessor unsafe references. The report generator generates a report of the multiprocessor unsafe references. Also, a checker that dynamically determines where instructions and data result in domain violations when run on a multiprocessor system. The checker includes an interface to a table of purportedly microprocessor safe references by the instructions and data outside of their domains. The table preferably includes the domains to which the references are supposed to refer. The checker also includes a reference tracker that tracks references made by the instructions and data, and a comparator that determines, when a reference in the table of purportedly microprocessor safe references is encountered during execution of the instructions and data, if the reference is actually to a domain to which that reference is supposed to refer.
Owner:NETWORK APPLIANCE INC
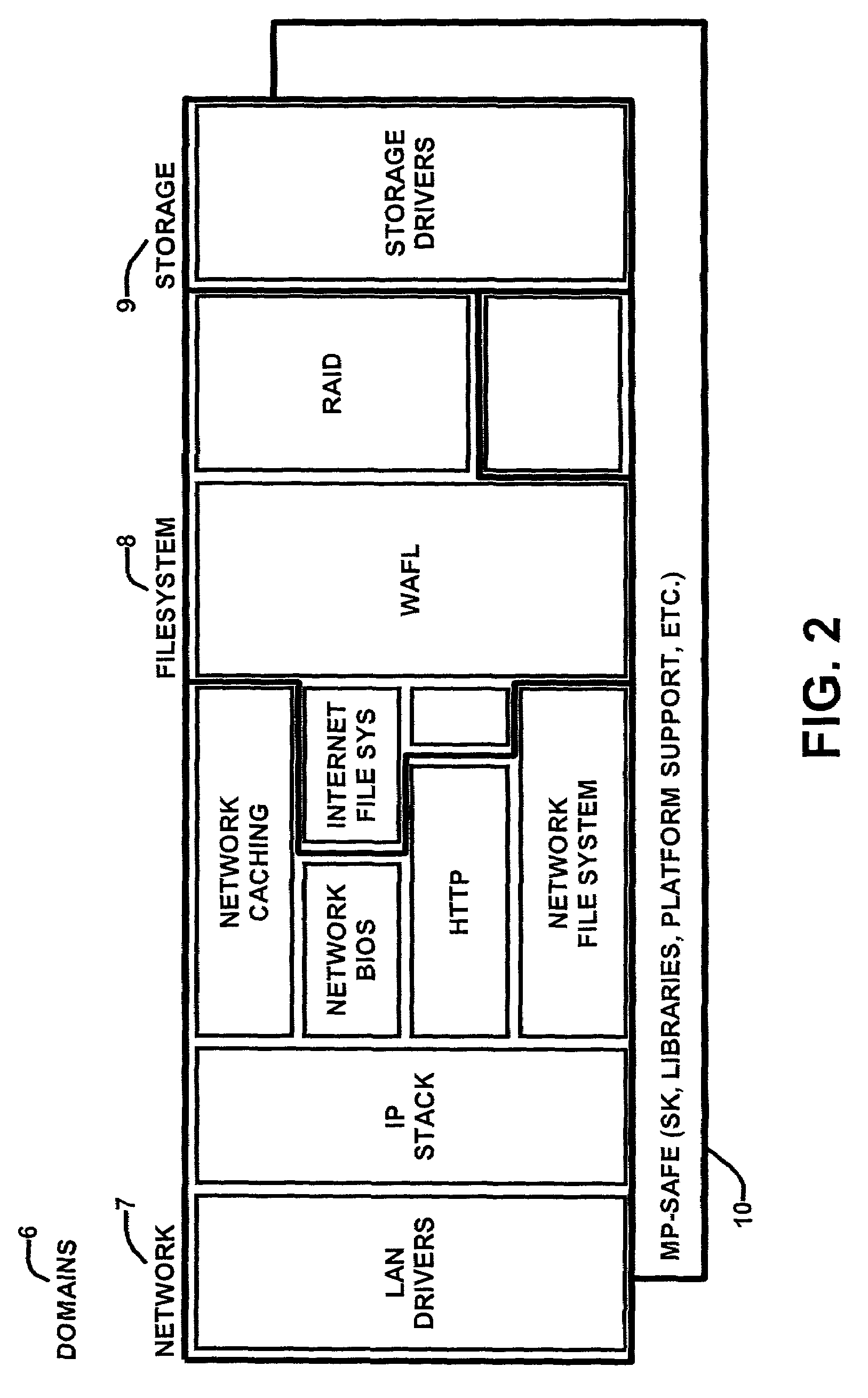
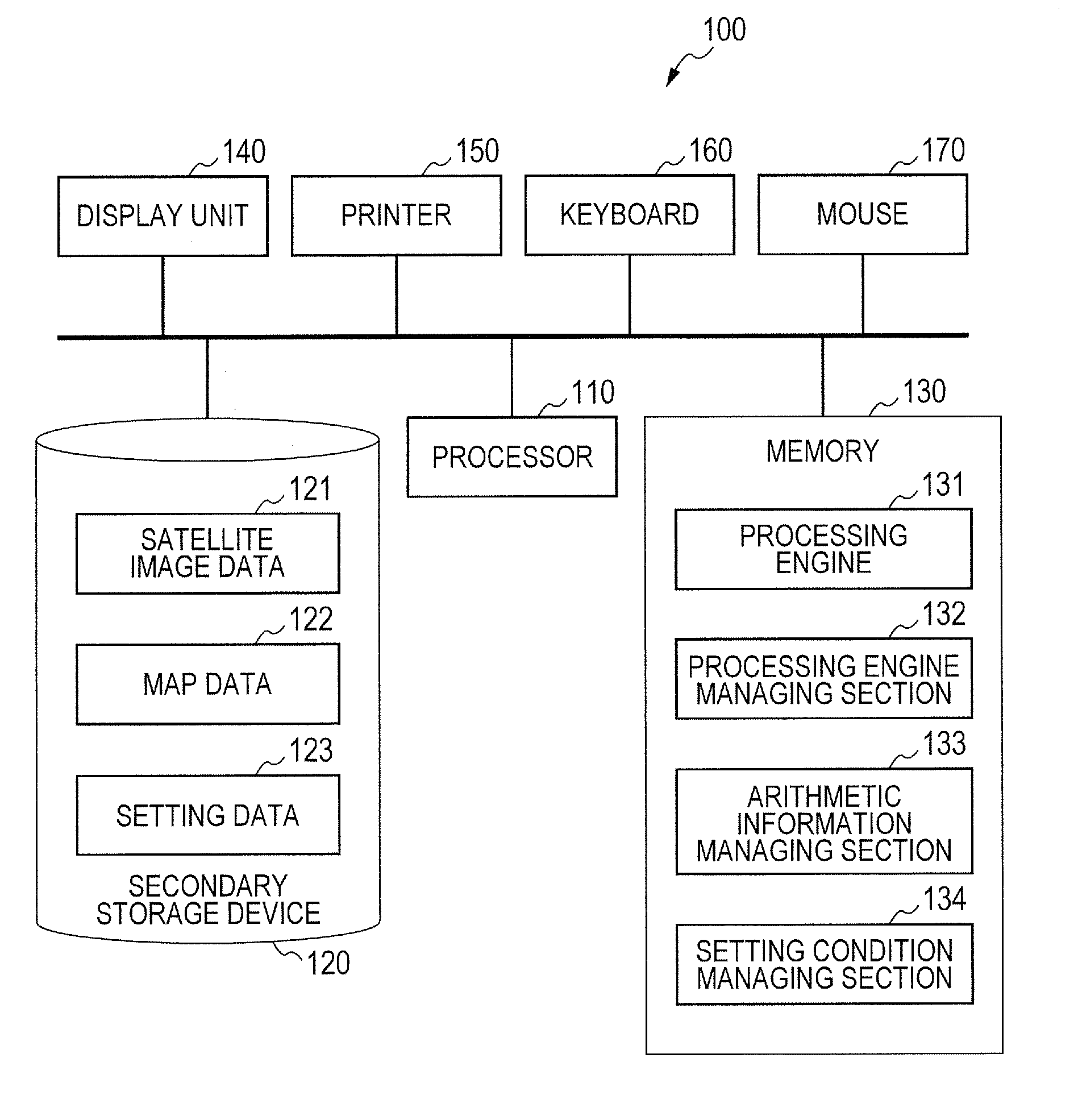
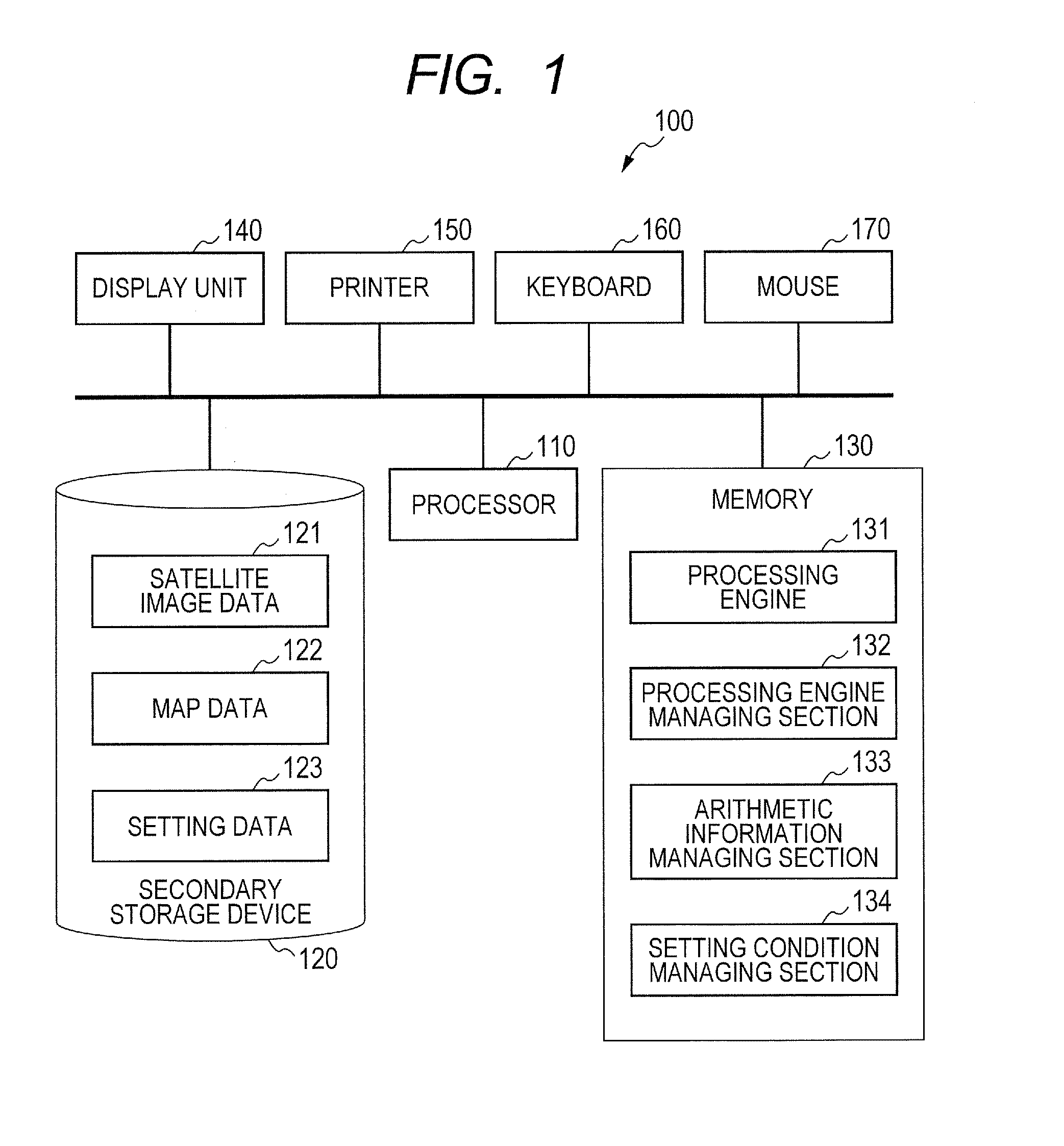
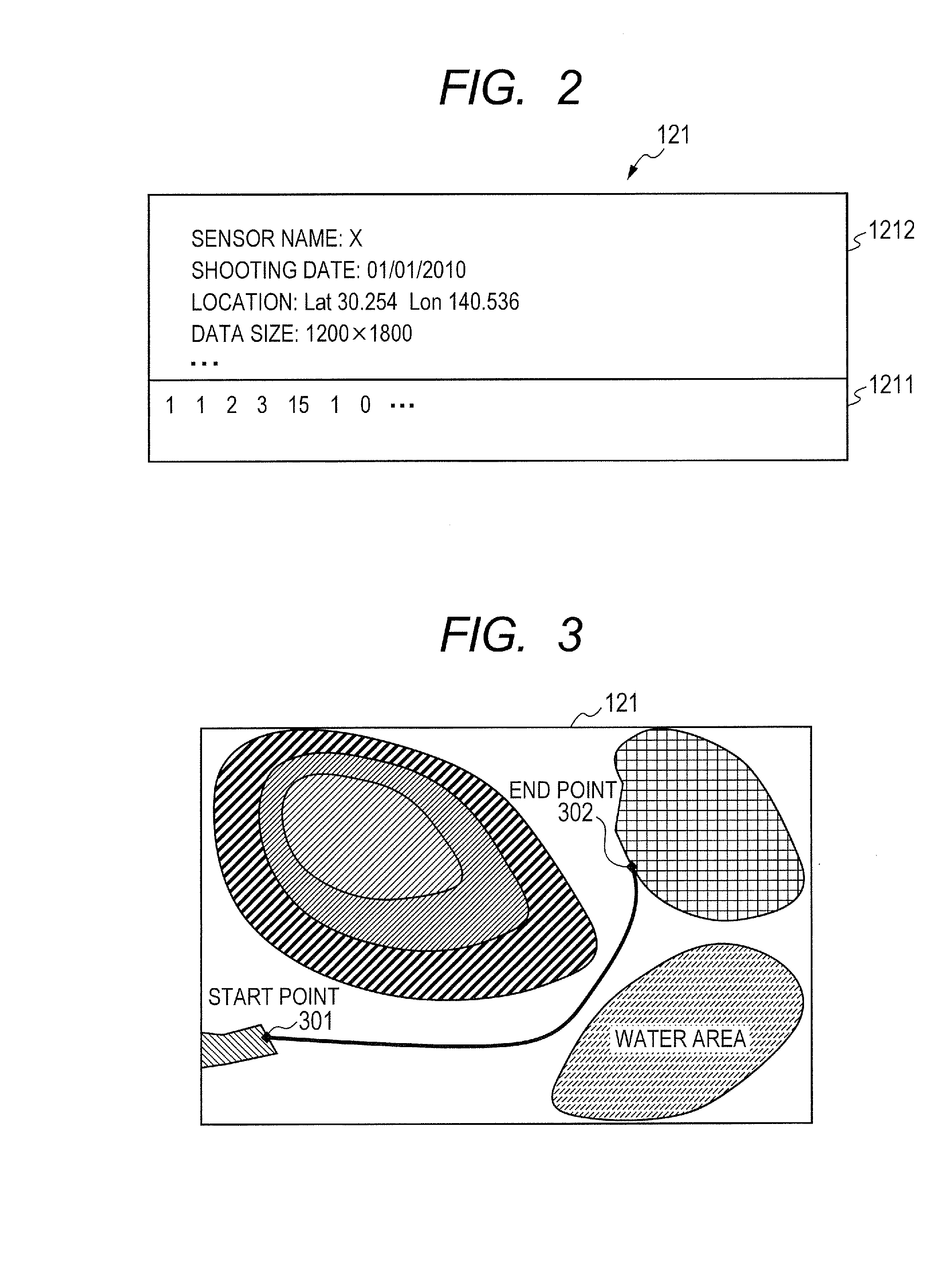
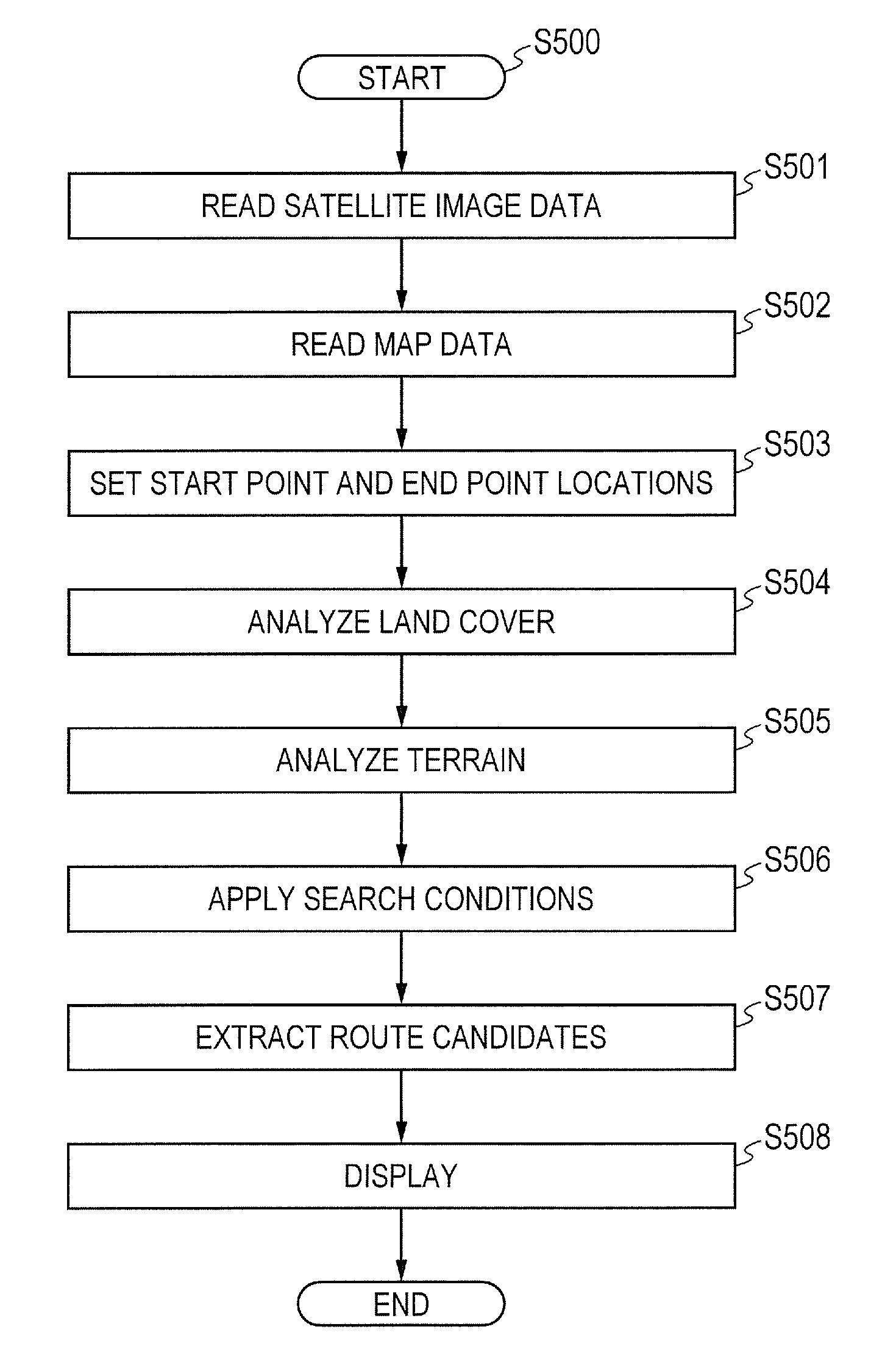
Route generation system, route generation method, and program
InactiveUS20130013204A1Instruments for road network navigationNavigational calculation instrumentsState dependentPath generation
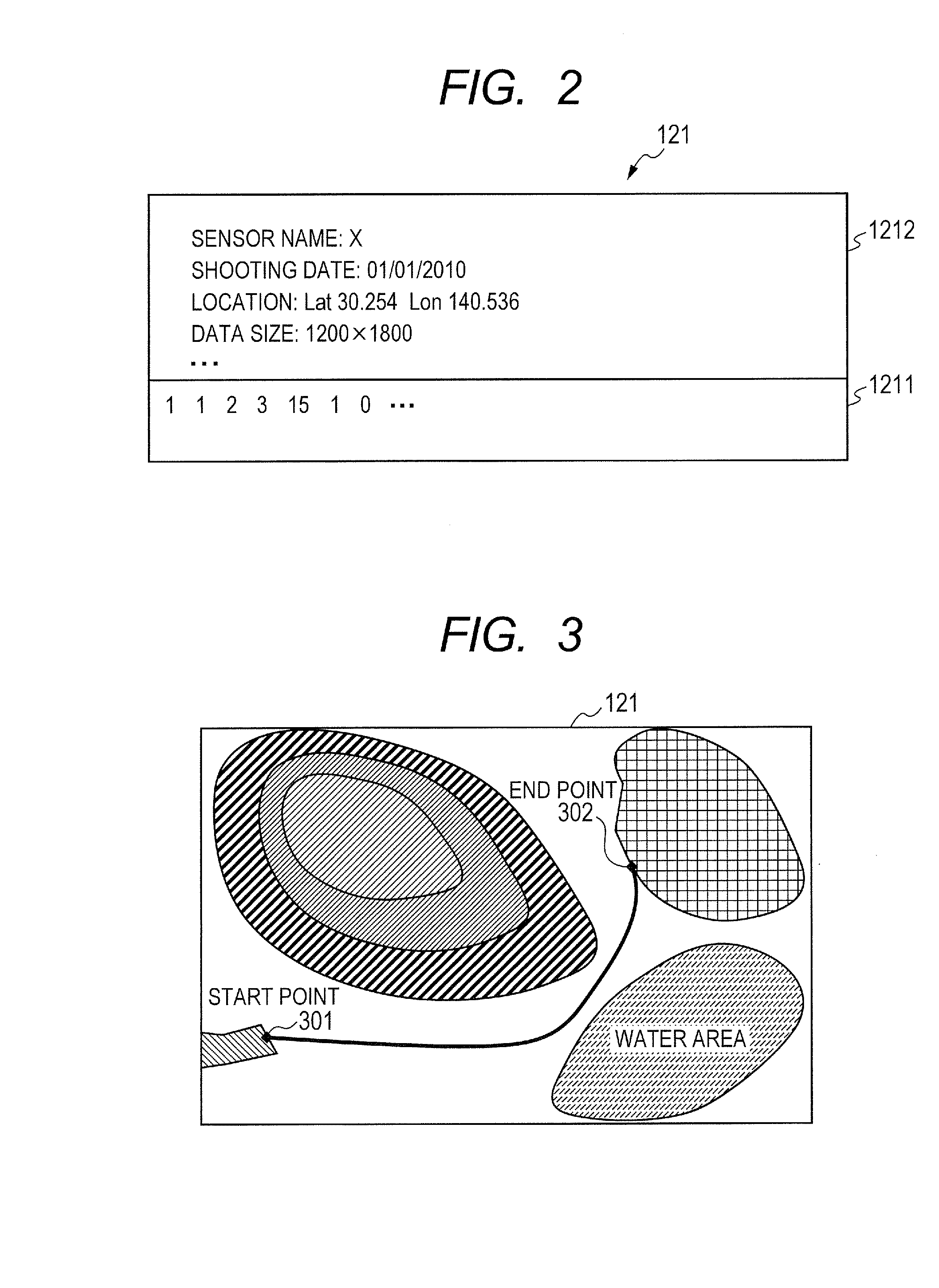
Provided is a route generation device capable of generating an appropriate route. A route generation system of one embodiment of the present invention includes: analysis sections that analyze aerial image data to identify the land state of an area included in an aerial image; a storage section that stores traffic cost information associating a traffic-cost coefficient indicating traffic difficulty with the land state; a route searching section that calculates traffic costs of a plurality of routes from a start point to an end point by referring to the analysis results of the analysis sections and the traffic cost information and that determines a route candidate to be used from the start point to the end point based on the calculation results.
Owner:HITACHI LTD
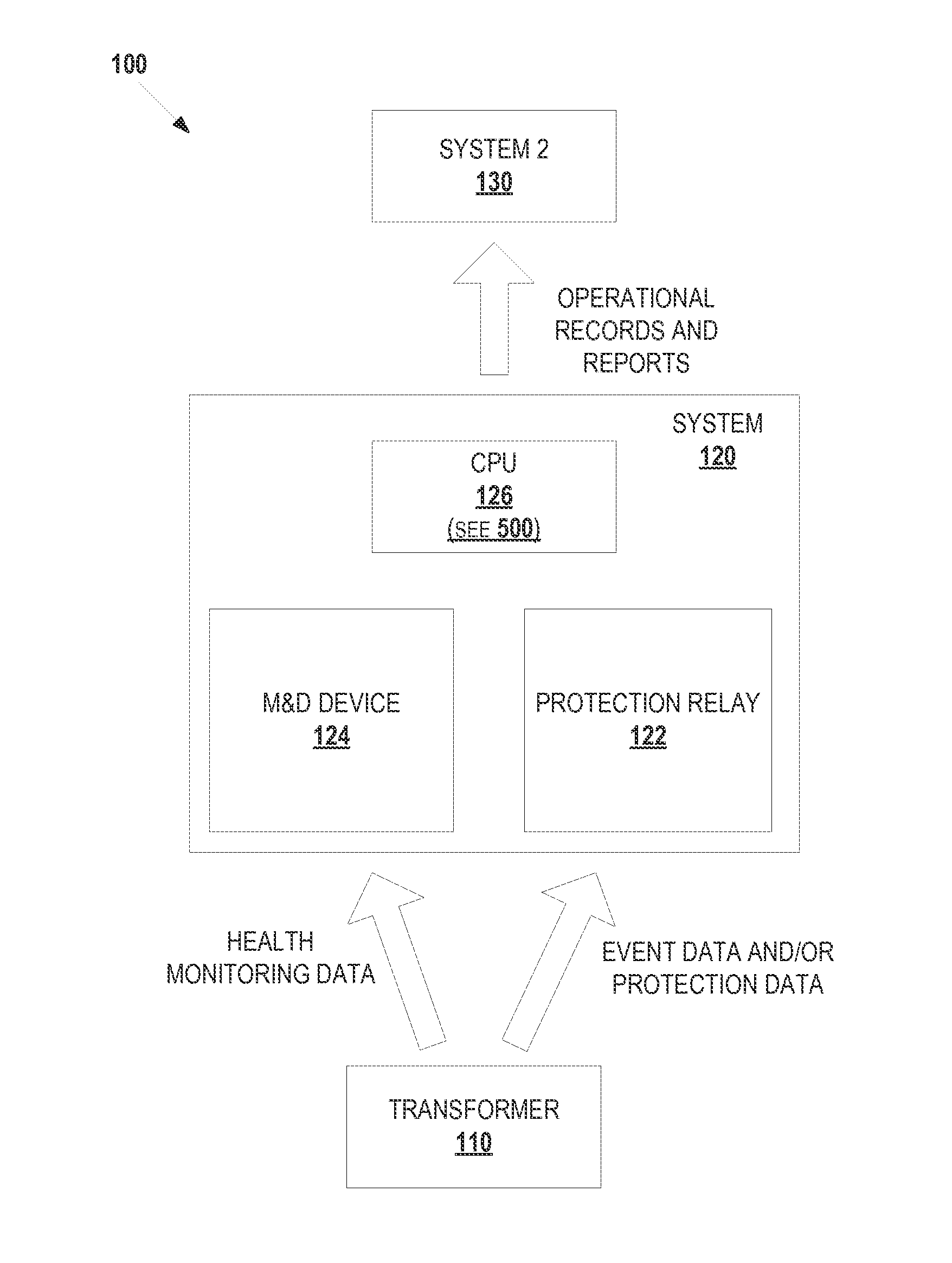
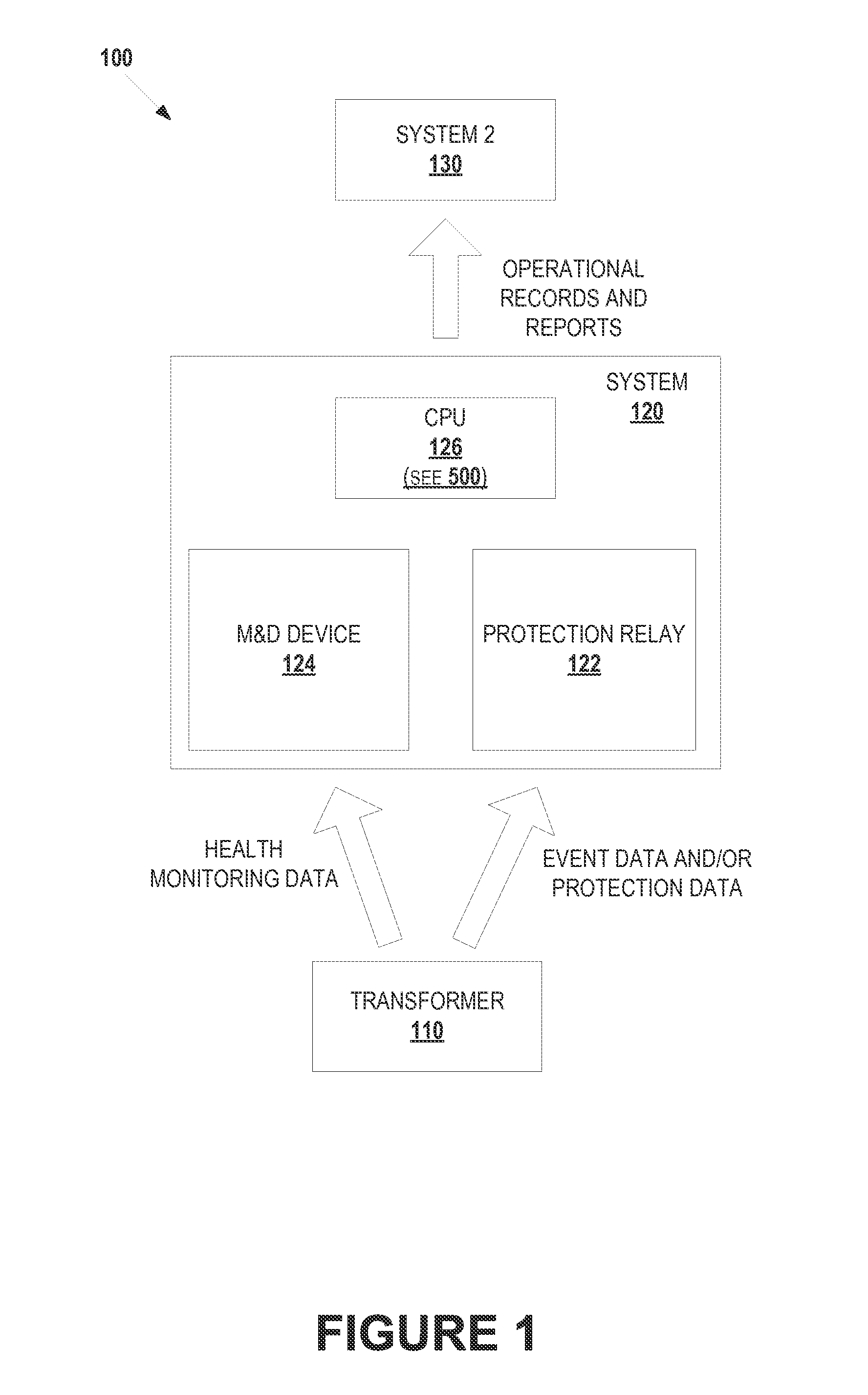
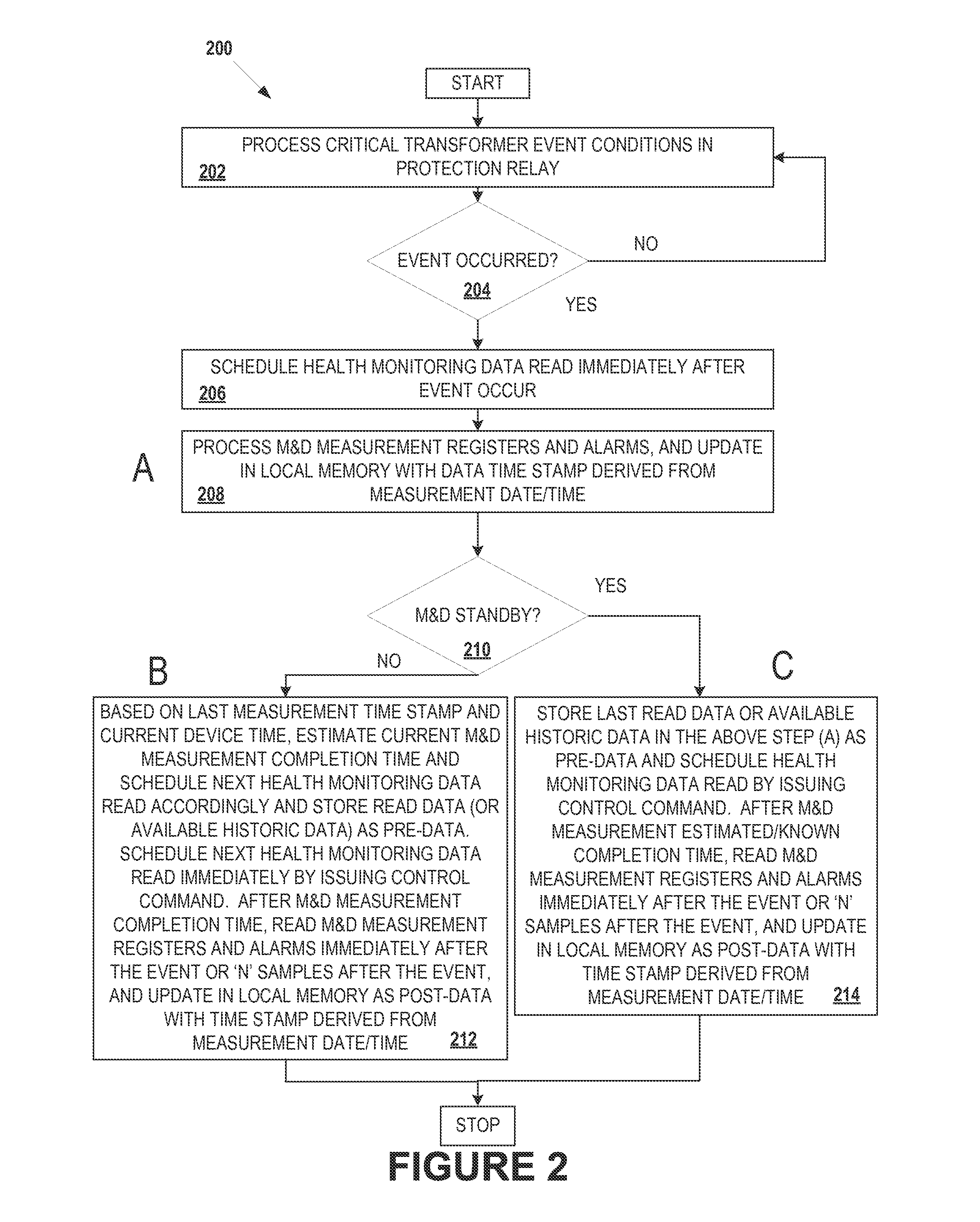
Integrated transformer health monitoring architecture
InactiveUS20160327600A1Emergency protective circuit arrangementsForecastingTransformerReference analysis
Embodiments of the present disclosure relate to an integrated transformer health monitoring architecture and associated processes. In one embodiment, a method can provide: receiving protection data of a transformer from the at least one protection relay device; receiving health monitoring data of the transformer from the at least one monitoring and diagnostics (M&D) device; analyzing the received protection data and the received health monitoring data using a common time reference; monitoring, diagnosing, analyzing, or predicting a failure of the transformer based at least in part on analysis of the received protection data and the received health monitoring data; and generating an instruction to modify operation of the transformer in response to monitoring, diagnosing, analyzing, or predicting a failure of the transformer.
Owner:GENERAL ELECTRIC CO
Route generation system, route generation method, and program
InactiveUS8880345B2Instruments for road network navigationNavigational calculation instrumentsState dependentPath generation
Owner:HITACHI LTD
Landslide monitoring system and monitoring method thereof
ActiveCN104596459AEfficient monitoring of reactionsImprove precisionMeasurement devicesTransmission systemsMonitoring systemLandslide
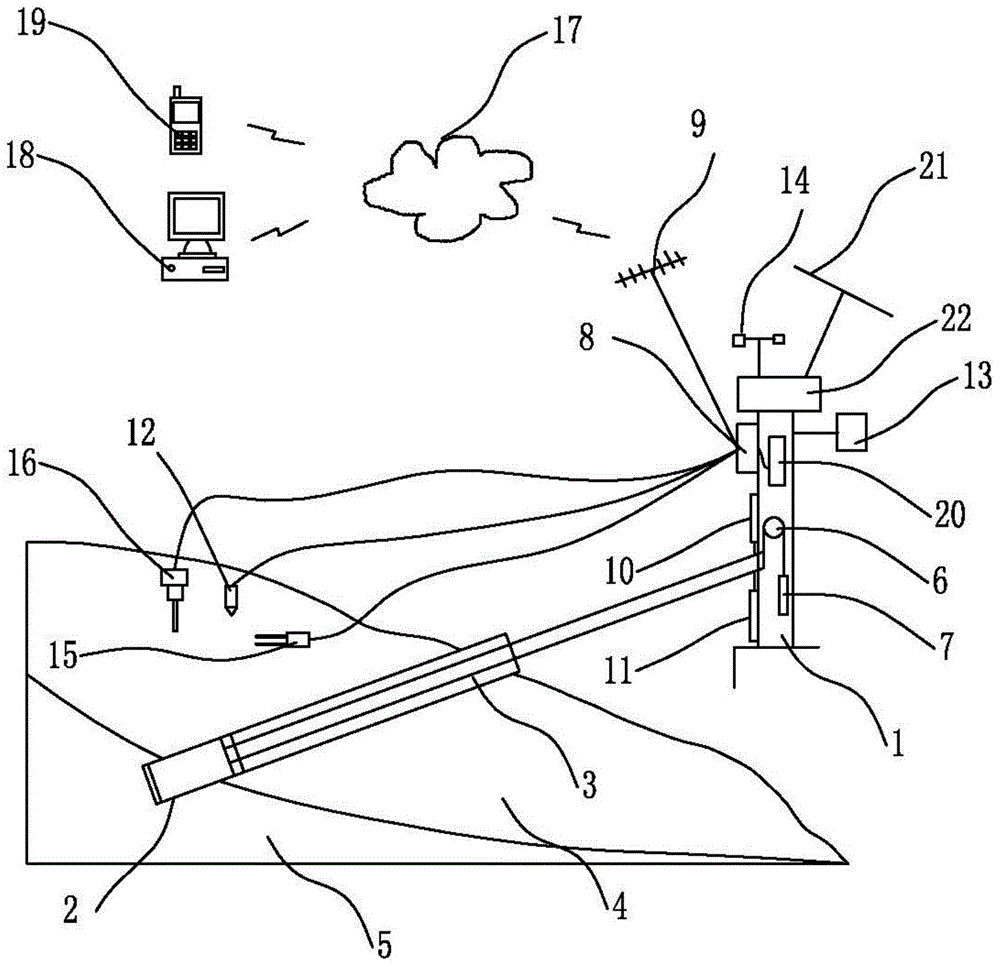
The invention discloses a landslide monitoring system and a monitoring method thereof. The landslide monitoring system adopts a displacement monitoring method to monitor landslide displacement, meanwhile is provided with a conventional linear displacement sensor for monitoring large linear displacement and a micro-metering linear displacement sensor for monitoring linear micro-displacement, ensures high monitoring accuracy while obtaining efficient monitoring response, controls working states of two displacement sensors through a control device, can be adjusted to adopt different monitoring modes so as to adapt to different monitoring environments and ensure monitoring in the most appropriate mode and ensures efficient, rapid and accurate monitoring. According to the corresponding monitoring method, an environment device is utilized to monitor the surrounding annular situation in real time, and reflection on different environments is timely made for reference analysis of the control device, conduction of targeted operation control and ensuring of the timely and appropriate monitoring mode.
Owner:CHINA NUCLEAR IND HUATAI CONSTR
Self-encoding sensor with microspheres
InactiveUS20050196317A1Bioreactor/fermenter combinationsBiological substance pretreatmentsSensor arrayFiber bundle
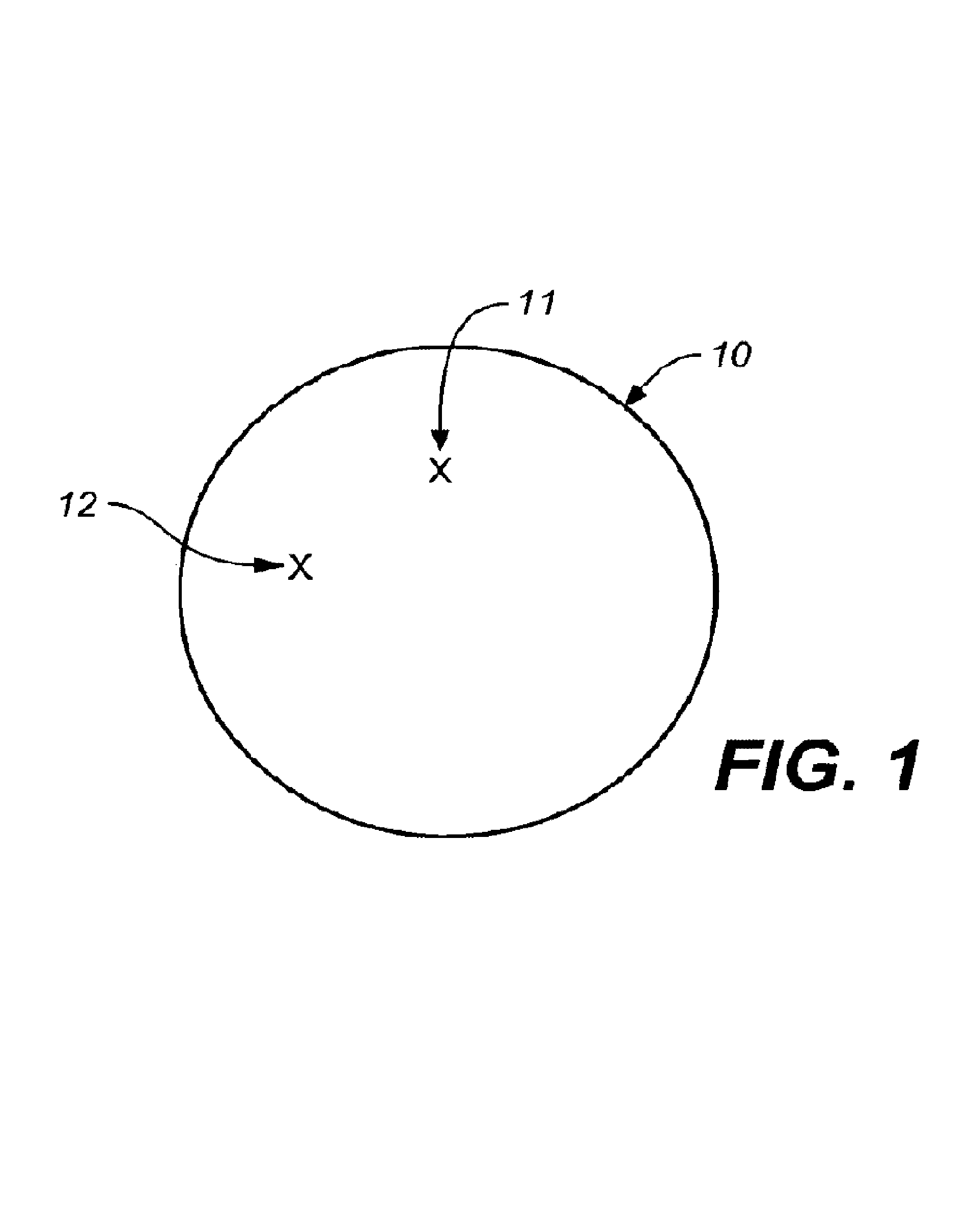
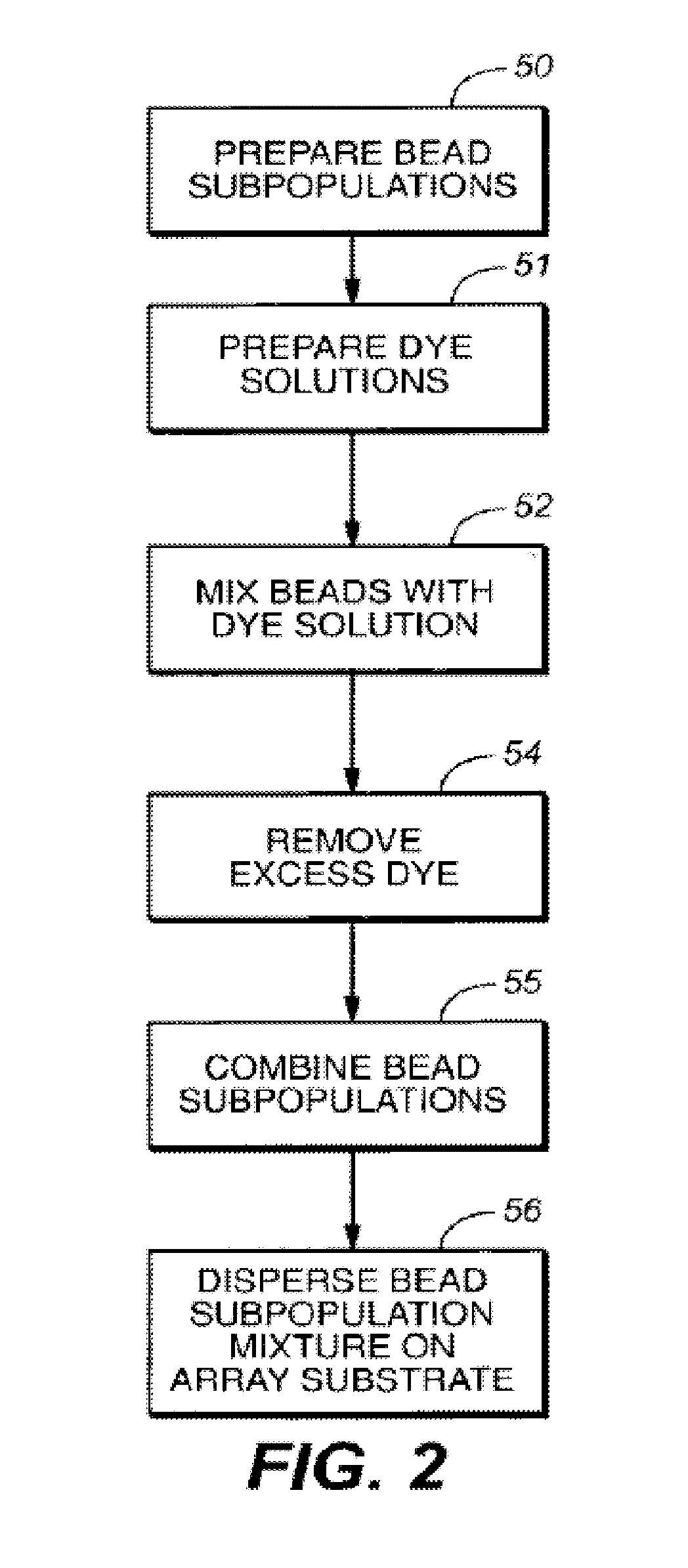
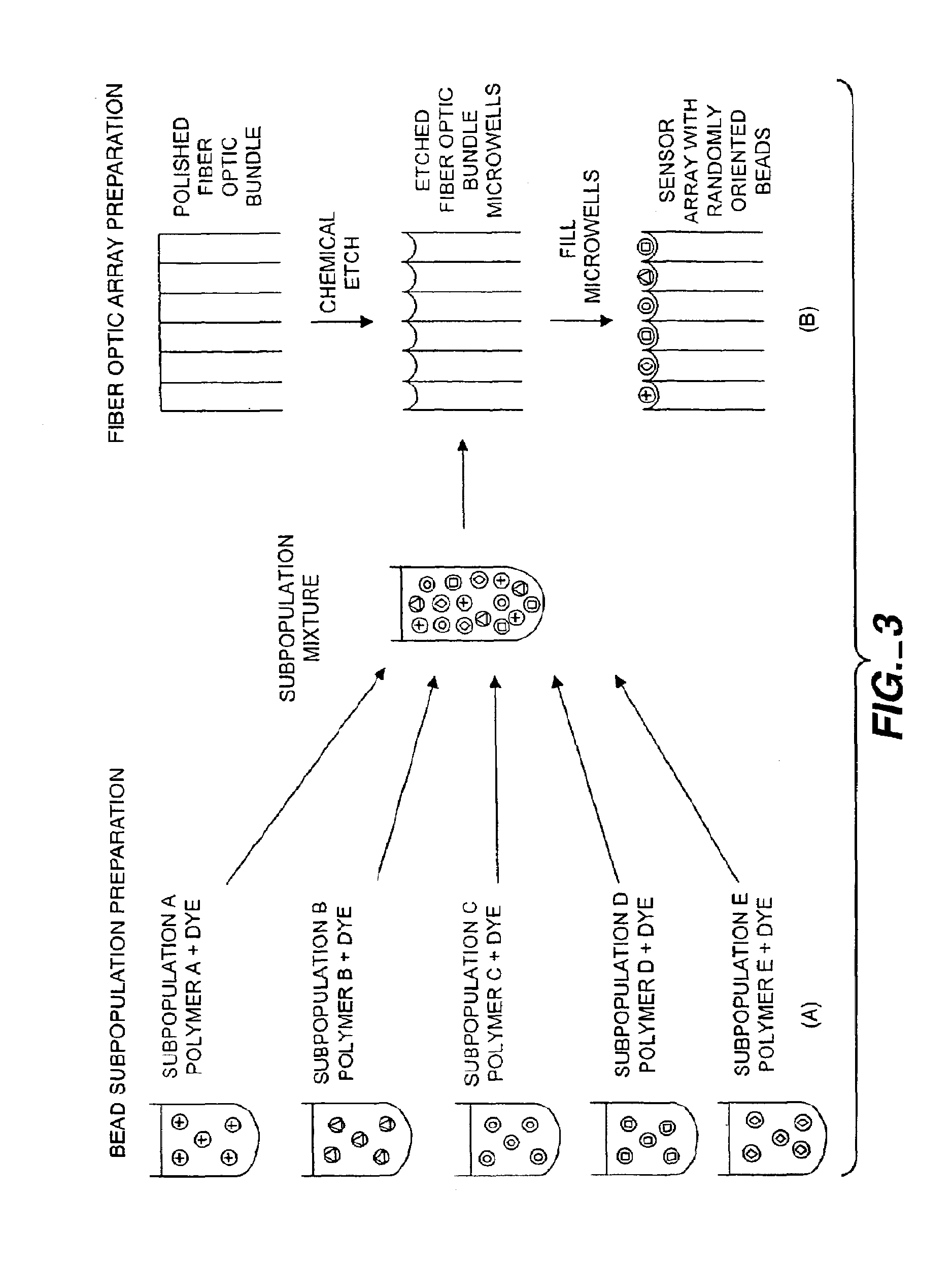
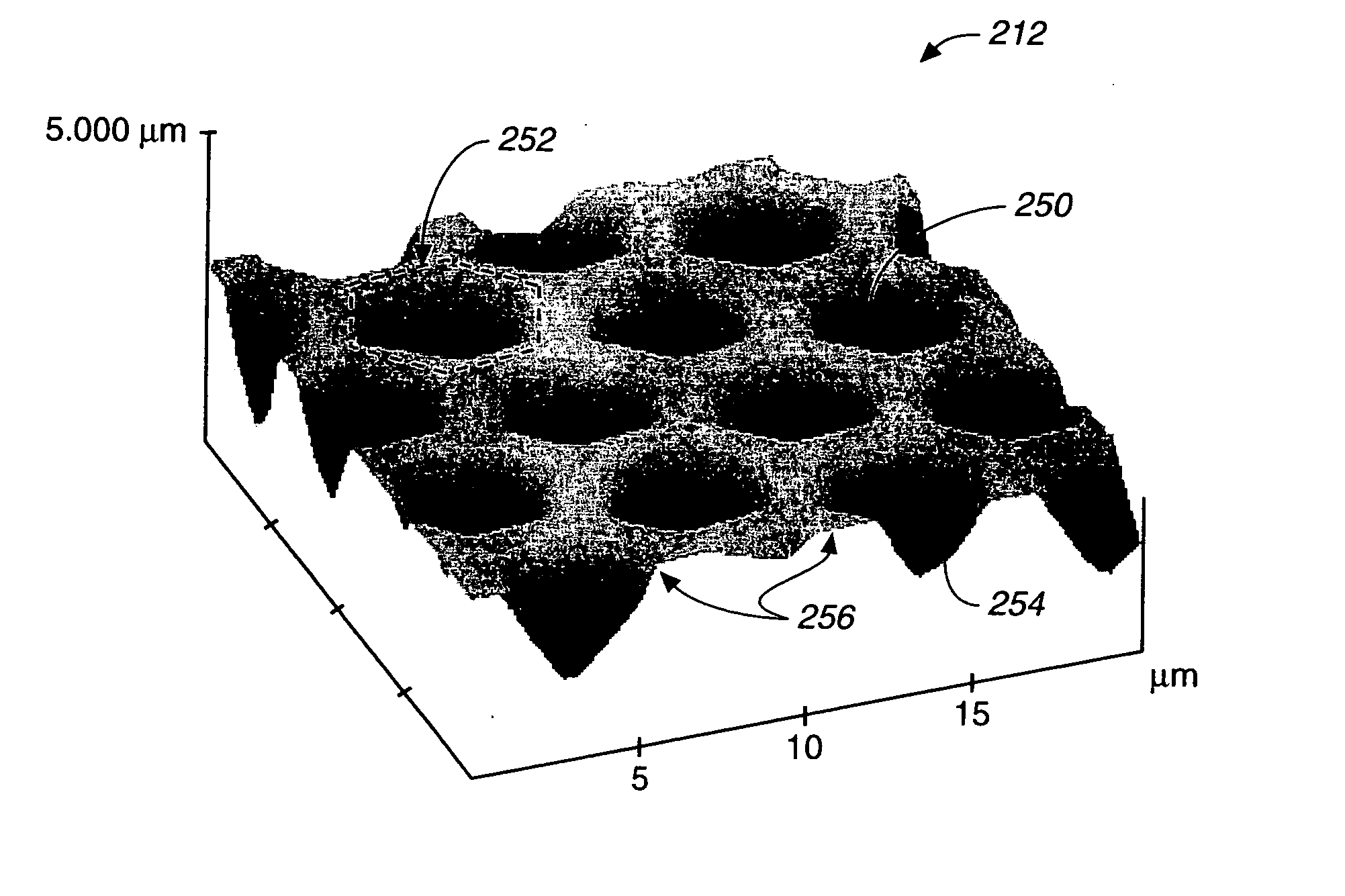
A microsphere-based analytic chemistry system is disclosed in which self-encoding microspheres having distinct characteristic optical response signatures to specific target analytes may be mixed together while the ability is retained to identify the sensor type and location of each sensor in a random dispersion of large numbers of such sensors in a sensor array using an optically interrogatable encoding scheme. An optical fiber bundle sensor is also disclosed in which individual microsphere sensors are disposed in microwells at a distal end of the fiber bundle and are optically coupled to discrete fibers or groups of fibers within the bundle. The identities of the individual sensors in the array are self-encoded by exposing the array to a reference analyte while illuminating the array with excitation light energy. A single sensor array may carry thousands of discrete sensing elements whose combined signal provides for substantial improvements in sensor detection limits, response times and signal-to-noise ratios.
Owner:TRUSTEES OF TUFTS COLLEGE
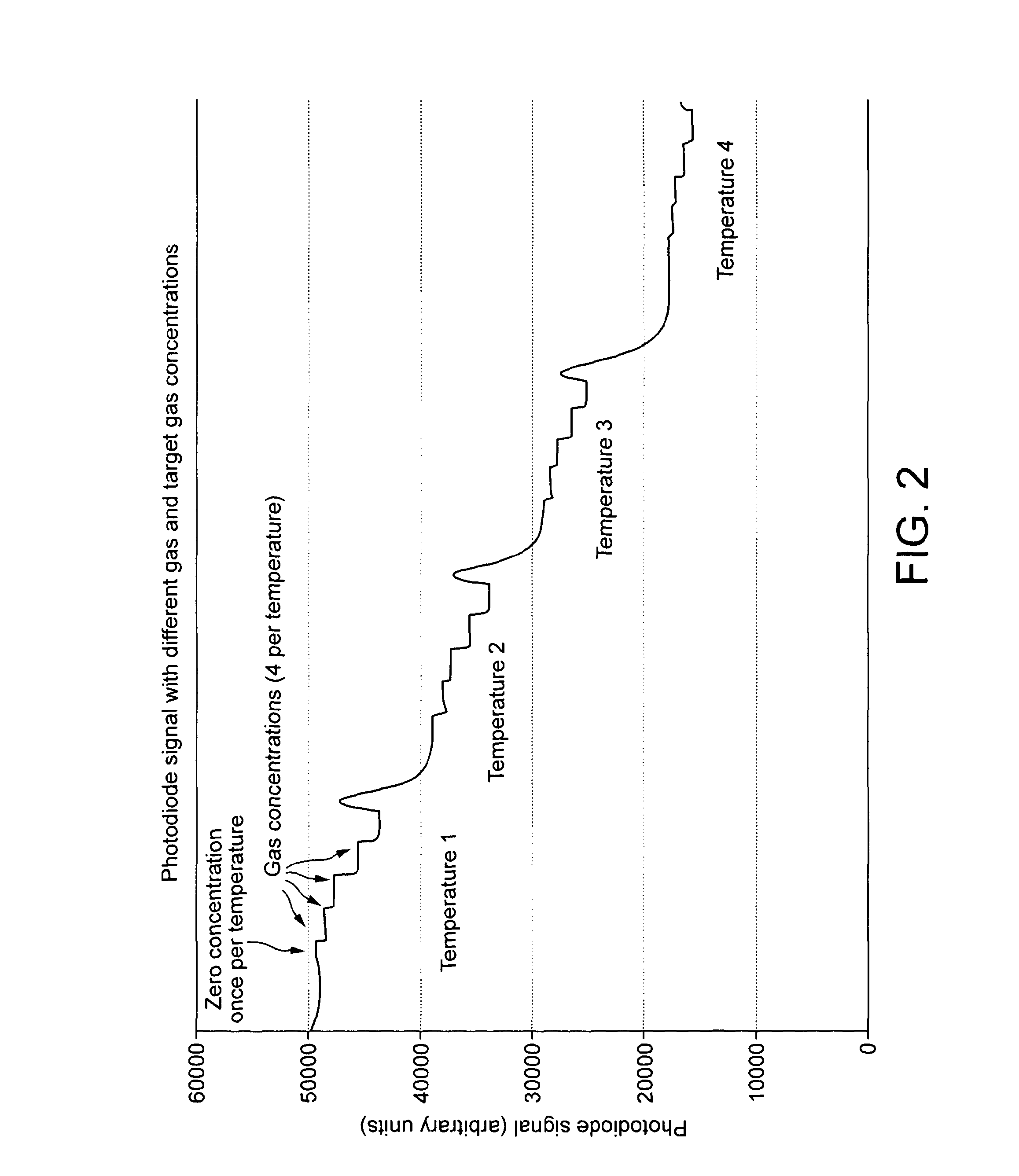
Temperature calibration methods and apparatus for optical absorption gas sensors, and optical absorption gas sensors thereby calibrated
ActiveUS20130301052A1Affect sensitivityColor/spectral properties measurementsGas analyser calibrationAnalyteOpto electronic
An optical absorption gas sensor has an LED light source and a photodiode light detector, a temperature measuring device for measuring the LED temperature and a temperature measuring device for measuring the photodiode temperature. The sensor is calibrated by measuring the response of photodiode current at zero analyte gas concentration and at a reference analyte gas concentration. From these measurement, calibration data taking into account the effect of photodiode temperature on the sensitivity of the photodiode and, independently, the effect of changes in the spectrum of light output by the LED on the light detected by the photodiode with LED temperature can be obtained. Calibration data is written to memory in the gas sensor and in operation of the gas sensor, the output is compensated for both LED and photodiode temperature. The LED and photodiode can therefore be relatively far apart and operate at significantly different temperatures allowing greater freedom of optical pathway design.
Owner:GAS SENSING SOLUTIONS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com