Medicament compound containing hyaluronic acid and beta-aescine sodium
A technology of sodium aescinate and hyaluronic acid, applied to tissue swelling, a pharmaceutical composition containing hyaluronic acid and sodium β-escinate, the application field of medicine, can solve articular cartilage damage, increase joint puncture Infection probability and other issues, to achieve the effect of delaying the curative effect, quick onset of effect, and reducing complications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
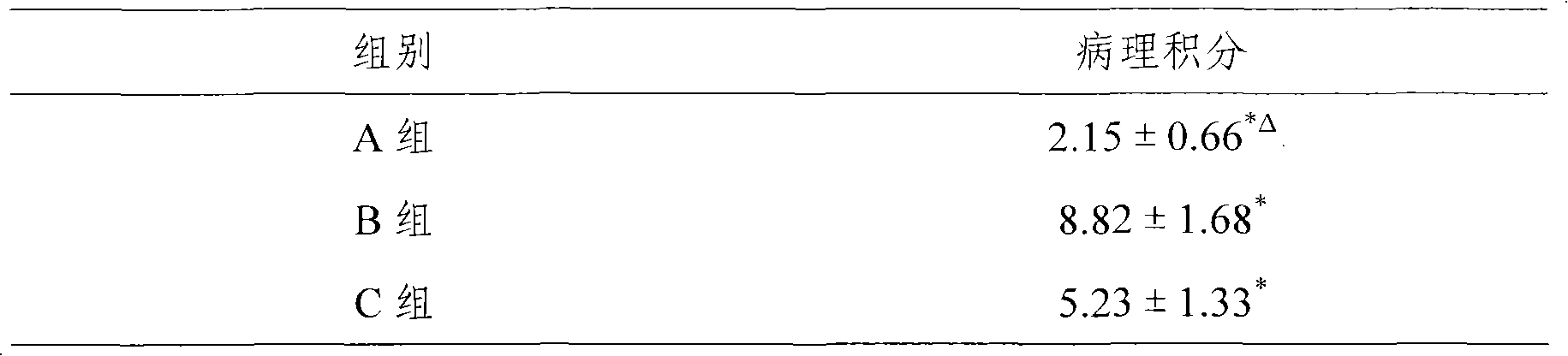
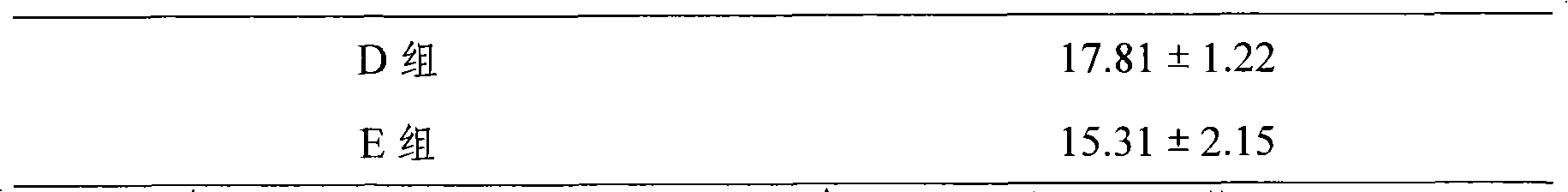
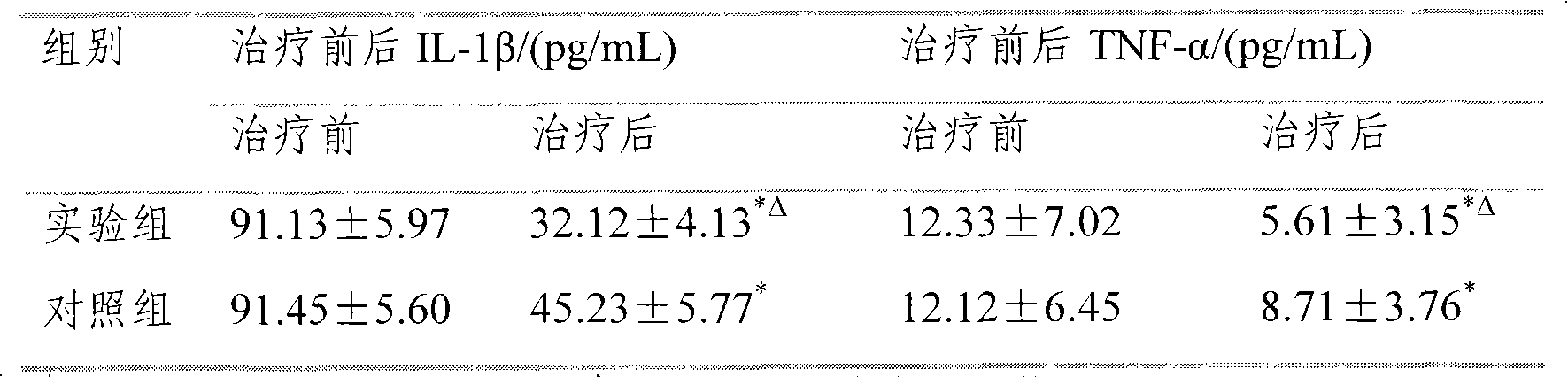
Embodiment 1-6
[0015] Composition and dosage
[0016] It can be obtained by subpackaging according to well-known conventional methods. When used, it can be injected directly into the joint cavity.
Embodiment 7
[0017] Example 7: Prepare a solution for intra-articular injection from the hyaluronic acid and β-escin sodium pharmaceutical composition of the present invention, and its usage and dosage are: 0.3 g of sodium hyaluronate, 0.3 g of sodium phylloside and the rest are phosphate buffer solution at pH 7.4, which are prepared and packaged according to known conventional methods. It can be directly injected into the joint cavity when used.
Embodiment 8
[0018] Example 8: Prepare an emulsion for intra-articular injection from the pharmaceutical composition of hyaluronic acid and β-escin sodium according to the present invention. 0.2 grams of sodium, 0.1 grams of lecithin, 0.3 grams of vitamin E, 0.1 grams of Tween 80, and the rest are phosphate buffer solution with a pH of 7.4, which are prepared and packaged according to known conventional methods. It can be directly injected into the joint cavity when used.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com