Artemisia pollen allergen vaccine and preparation method thereof
An allergen and pollen technology, applied in the directions of allergen antigen components, respiratory diseases, skin diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
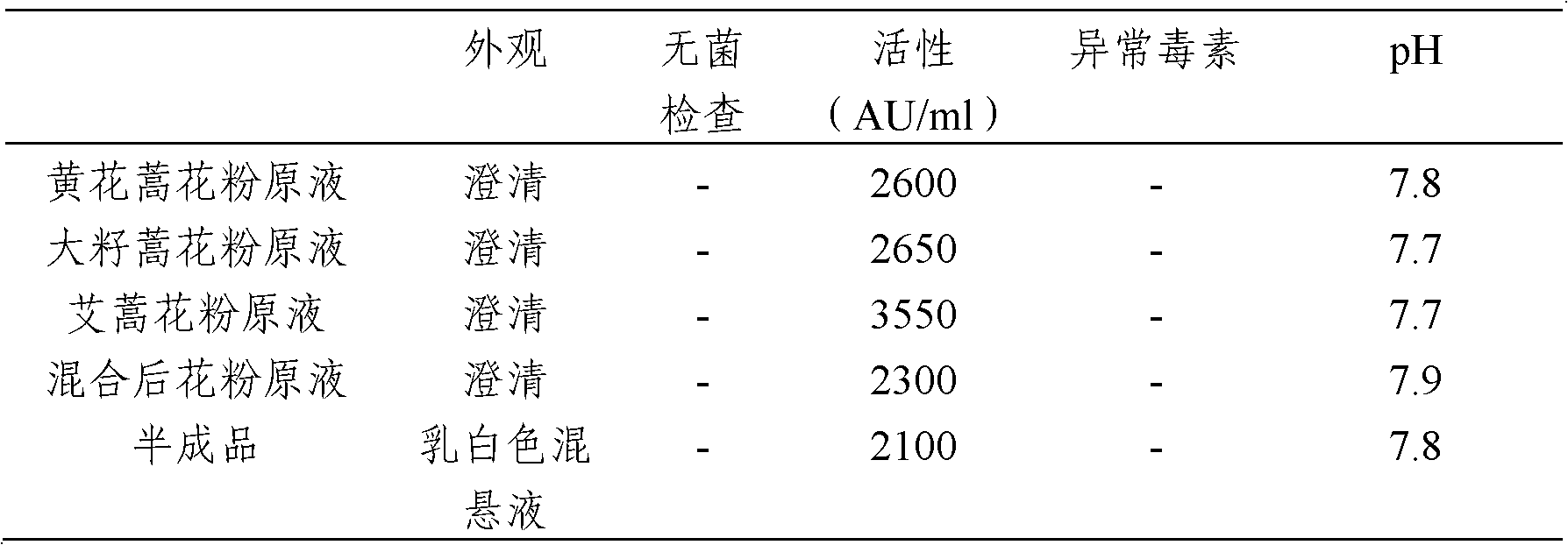
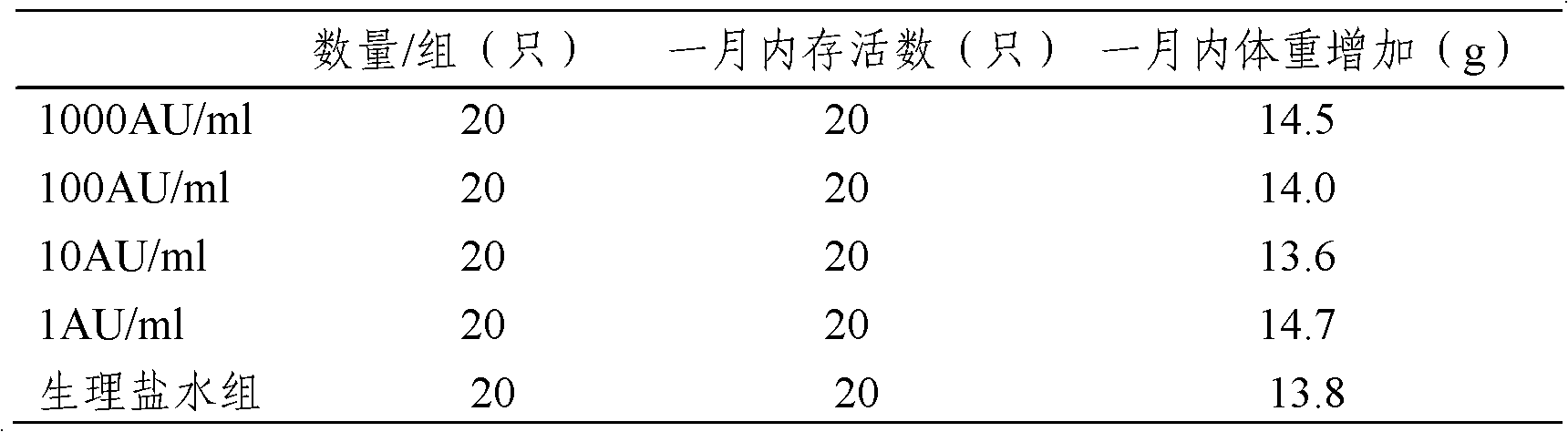
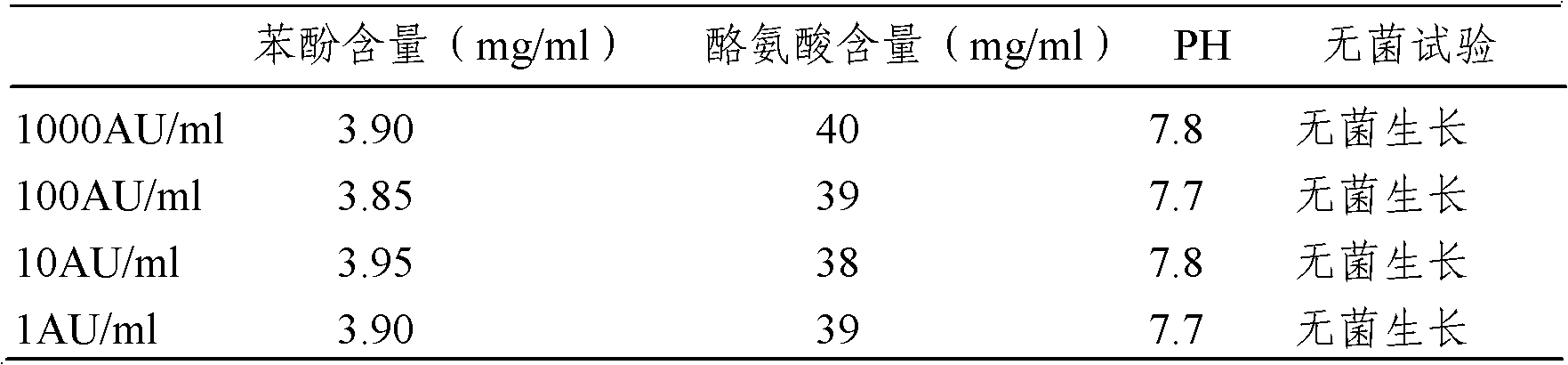
[0029] The following examples are used to illustrate the present invention, but are not intended to limit the scope of the present invention. Example 1 Preparation of Artemisia grandis pollen allergen stock solution
[0030] 1) The pollen of Artemisia grandis was collected mechanically (vacuum cleaner), and then impurities such as dust were removed using a 300-mesh sieve, and the collected pollen was tested for purity, morphological and molecular biological identification.
[0031] 2) Add the pollen obtained in 1) to acetone at a volume ratio of 1:5, and degrease under intermittent stirring at room temperature for 3 hours each time.
[0032] 3) Add 1000 g of degreased Artemisia grandis pollen to 25000 ml of 200 mM pH8.0 disodium hydrogen phosphate-sodium dihydrogen phosphate buffer at a feed ratio of 1:25 (W:V), and stir at 4°C for 24 hours.
[0033] 4) Centrifuge the extracted extract at 6000rpm at 4°C for 15min. Take the supernatant after centrifugation at 8000rpm at 4°C f...
Embodiment 2
[0037] Example 2 Preparation of Artemisia annua pollen allergen stock solution
[0038] 1) The pollen of Artemisia annua was collected mechanically (vacuum cleaner), and then impurities such as dust were removed using a 300-mesh sieve, and the collected pollen was tested for purity, morphological and molecular biological identification.
[0039] 2) Add the pollen obtained in 1) to acetone at a volume ratio of 1:5, and degrease under intermittent stirring at room temperature for 3 hours each time.
[0040] 3) Add 1,000 g of defatted Artemisia annua pollen to 25,000 ml of 200 mM pH 8.0 disodium hydrogen phosphate-sodium dihydrogen phosphate buffer at a feed ratio of 1:25 (W:V), and stir at 4° C. for 24 hours.
[0041] 4) Centrifuge the extracted extract at 6000rpm at 4°C for 15min. Take the supernatant after centrifugation at 8000rpm at 4°C for 30min.
[0042] 5) Clarify and filter the extract after extraction with an 8 μm filter membrane, and then filter the crude extract thr...
Embodiment 3
[0045] Example 3: Preparation of mugwort pollen allergen stock solution
[0046] 1) Collect mugwort pollen mechanically (vacuum cleaner), then use a 300-mesh sieve to remove dust and other impurities, and conduct purity testing, morphological and molecular biology identification on the collected pollen.
[0047] 2) Add the pollen obtained in 1) to acetone at a volume ratio of 1:5, stir intermittently at room temperature for degreasing, each time for 3 hours, until the supernatant acetone is colorless after degreasing, and dry the degreasing solid naturally until there is no smell of acetone After weighing.
[0048] 3) Add 1000 g of degreased mugwort pollen to 25000 ml of 200 mM disodium hydrogen phosphate-sodium dihydrogen phosphate buffer at a feed ratio of 1:25 (W:V), and stir at 4° C. for 24 hours.
[0049] 4) Centrifuge the extracted extract at 6000 rpm at 4° C. for 15 min. Take the supernatant at 8000rpm, centrifuge at 4°C for 30min and take the supernatant.
[0050] 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com