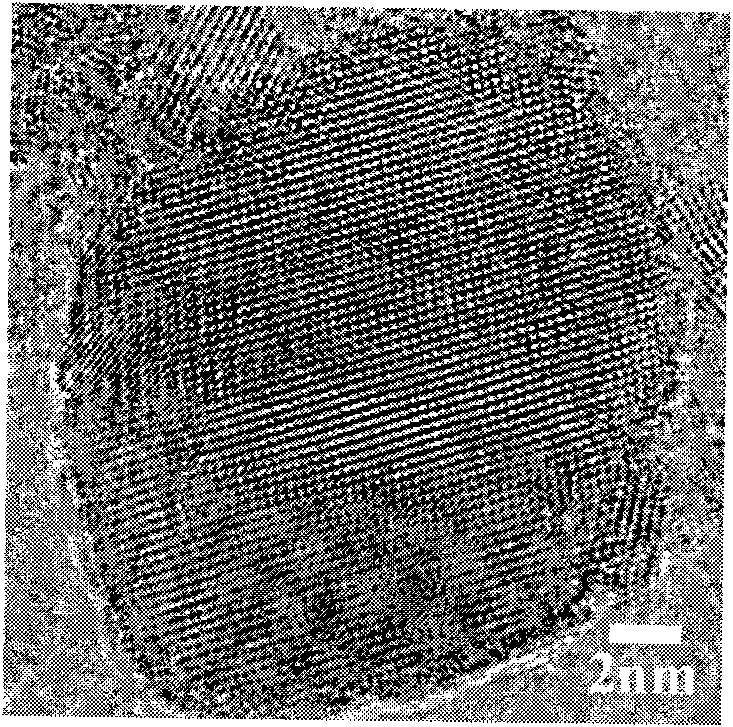
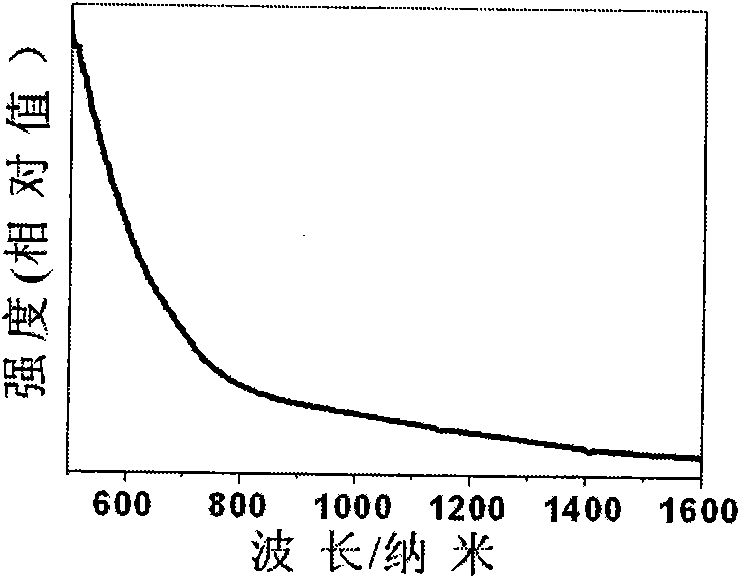
Preparation method of Cu2CdSnSe4 nano crystals
A technology of nanocrystals and reactants, applied in chemical instruments and methods, inorganic chemistry, non-metallic elements, etc., to achieve the effect of low cost, simple preparation method, and good crystallinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Add 80mmol oleylamine into the three-necked round-bottomed flask reactor, and sequentially add the reactant precursors 1mmol copper acetylacetonate, 0.5mmol cadmium acetate, 0.5mmol tin acetate and 2mmol selenium powder into the reaction flask, and rise in an argon atmosphere. High temperature until all precursors are dissolved. In an atmosphere of argon, the reaction temperature was increased to 300° C. for 15 minutes, and after the reaction was stopped, methanol was added to the cooled reaction product to cause the nanoparticles to settle. Finally, the nanocrystals were collected by centrifugation at 12,000 rpm for 3 minutes.
Embodiment 2
[0018] Add 20mmol oleylamine into the three-necked round-bottomed flask reactor, and sequentially add the reactant precursors 0.5mmol copper acetate, 0.25mmol cadmium acetate, 0.25mmol tin acetate and 1mmol trioctylphosphine selenium into the reaction flask, Increase the temperature in the atmosphere until the precursors are completely dissolved. In an atmosphere of argon, the reaction temperature was raised to 290° C. for 20 minutes, and after the reaction was stopped, methanol was added to the cooled reaction product to cause the nanoparticles to settle. Finally, the nanocrystals were collected by centrifugation at 10,000 rpm for 5 min.
Embodiment 3
[0020] Add 200mmol oleylamine into the three-necked round-bottomed flask reactor, and sequentially add the reactant precursors 10mmol copper acetylacetonate, 2.5mmol cadmium chloride, 2.5mmol tin acetate and 10mmol selenourea into the reaction flask, in an argon atmosphere Raise the temperature until all the precursors are dissolved. In an atmosphere of argon, the reaction temperature was increased to 280° C. for 35 minutes, and after the reaction was stopped, methanol was added to the cooled reaction product to cause the nanoparticles to settle. Finally, the nanocrystals were collected by centrifugation at 8000 rpm for 8 min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com