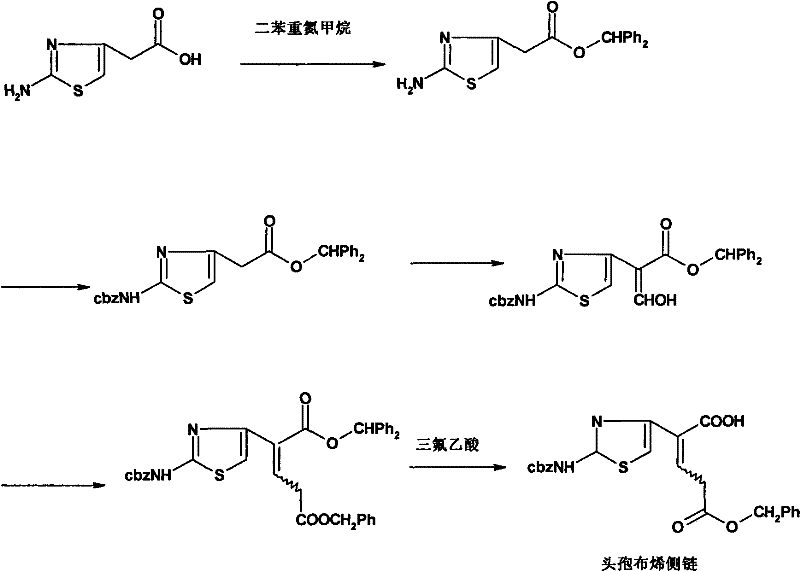
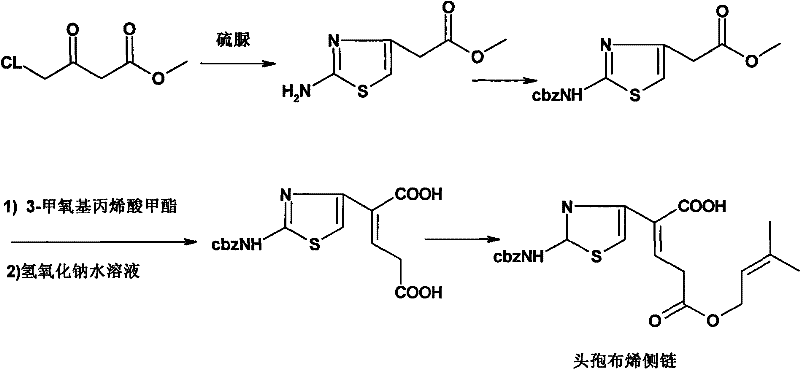
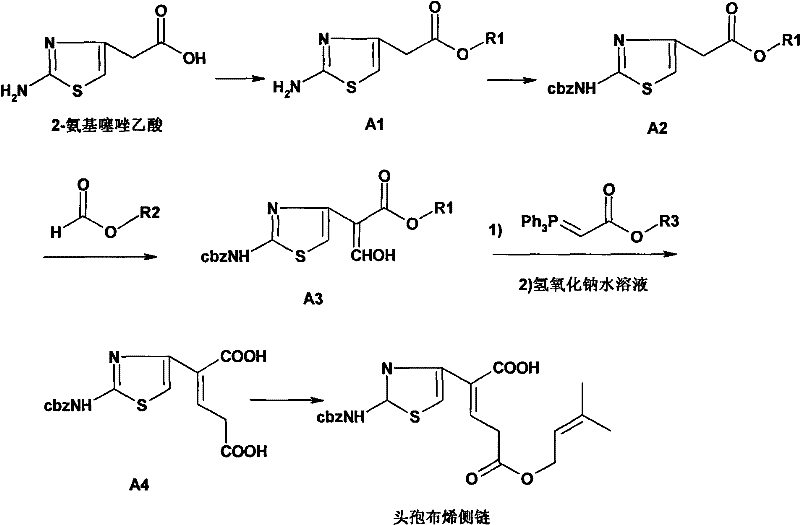
Synthesization technique for ceftibuten side chain
A ceftibuten side chain and synthesis process technology, applied in the direction of organic chemistry, can solve the problems of ceftibuten difficult to localize and process to industrialize, and achieve strong controllability, stable yield, and low yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] The present invention will be further described below in conjunction with specific embodiment:
[0030] Carboxyl protection. Take a 5000mL dry three-neck flask, add anhydrous methanol (1600mL, solvent and reactant) and 2-aminothiazoleacetic acid (250g, 1.59mol) under mechanical stirring, add concentrated sulfuric acid (8g, 0.08mol) after stirring evenly, stir and heat up Return to reflux and maintain for 48 hours, recover about 1300mL of methanol by distillation under normal pressure, slowly cool to room temperature with stirring, add 400mL of ethyl acetate:petroleum ether=1:4 mixed solution, precipitate solids under stirring until complete, filter with suction, filter cake Wash with petroleum ether and dry to obtain off-white solid: methyl 2-aminothiazole acetate, 223 g in total. Crude yield: 82%. mp: 124-126°C. 1 H-NMR (CDCl 3 ): δ: 3.54(s, 2H), 3.68(s, 3H), 5.57(s, 2H), 6.29(s, 1H); 13 C-NMR (CDCl 3 ): δ: 37.6, 52.5, 104.0, 145.1, 169.1, 171.4.
[0031] Amino ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com