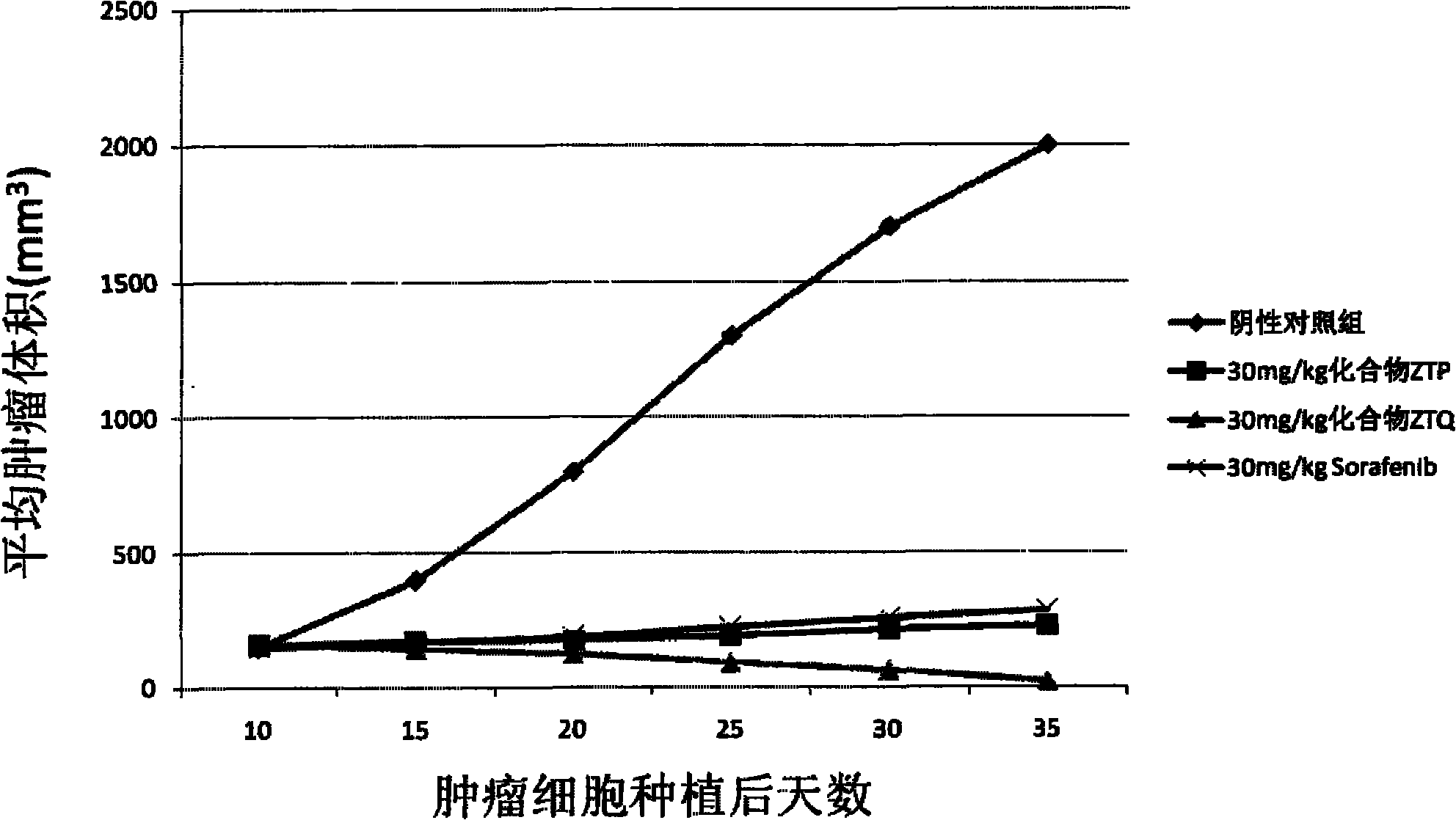
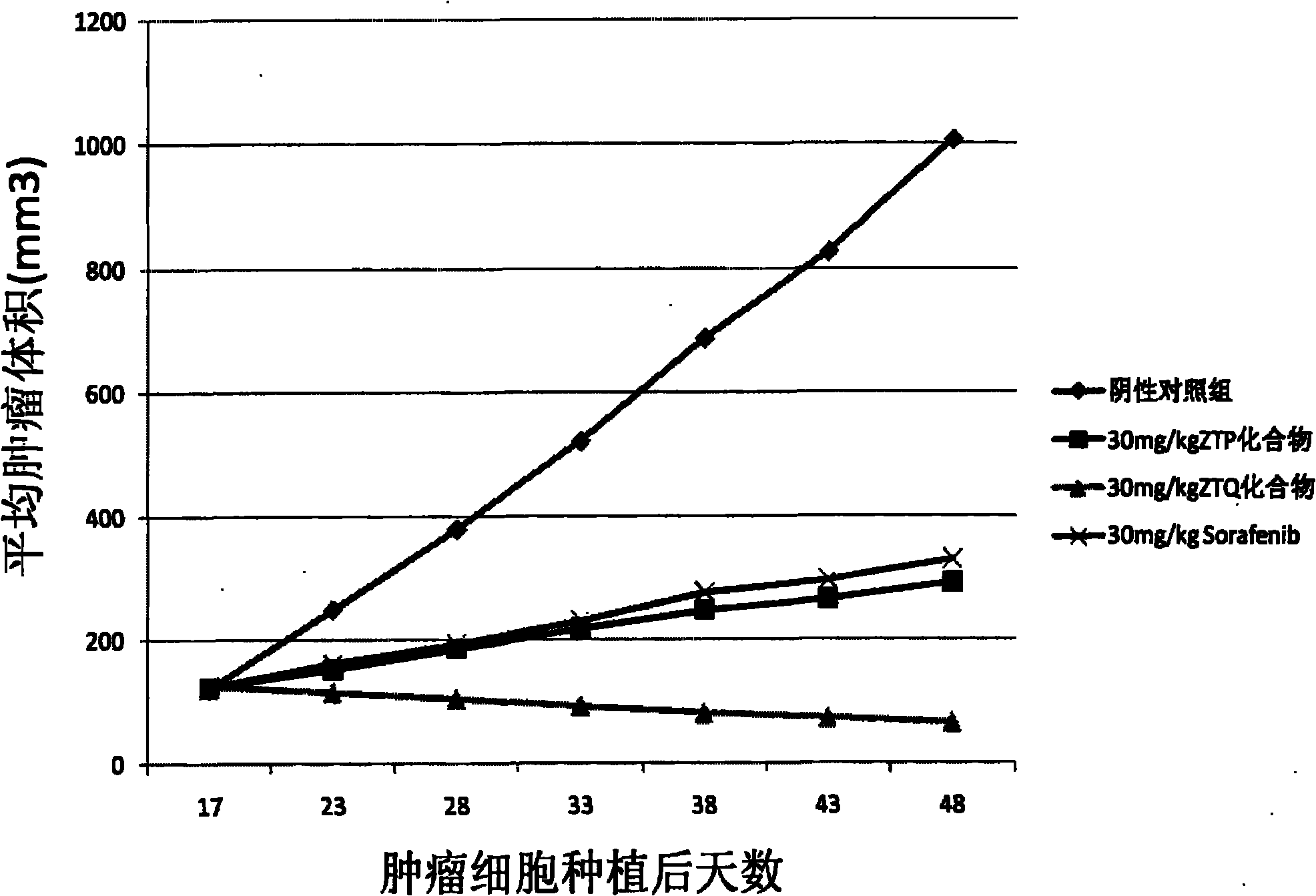
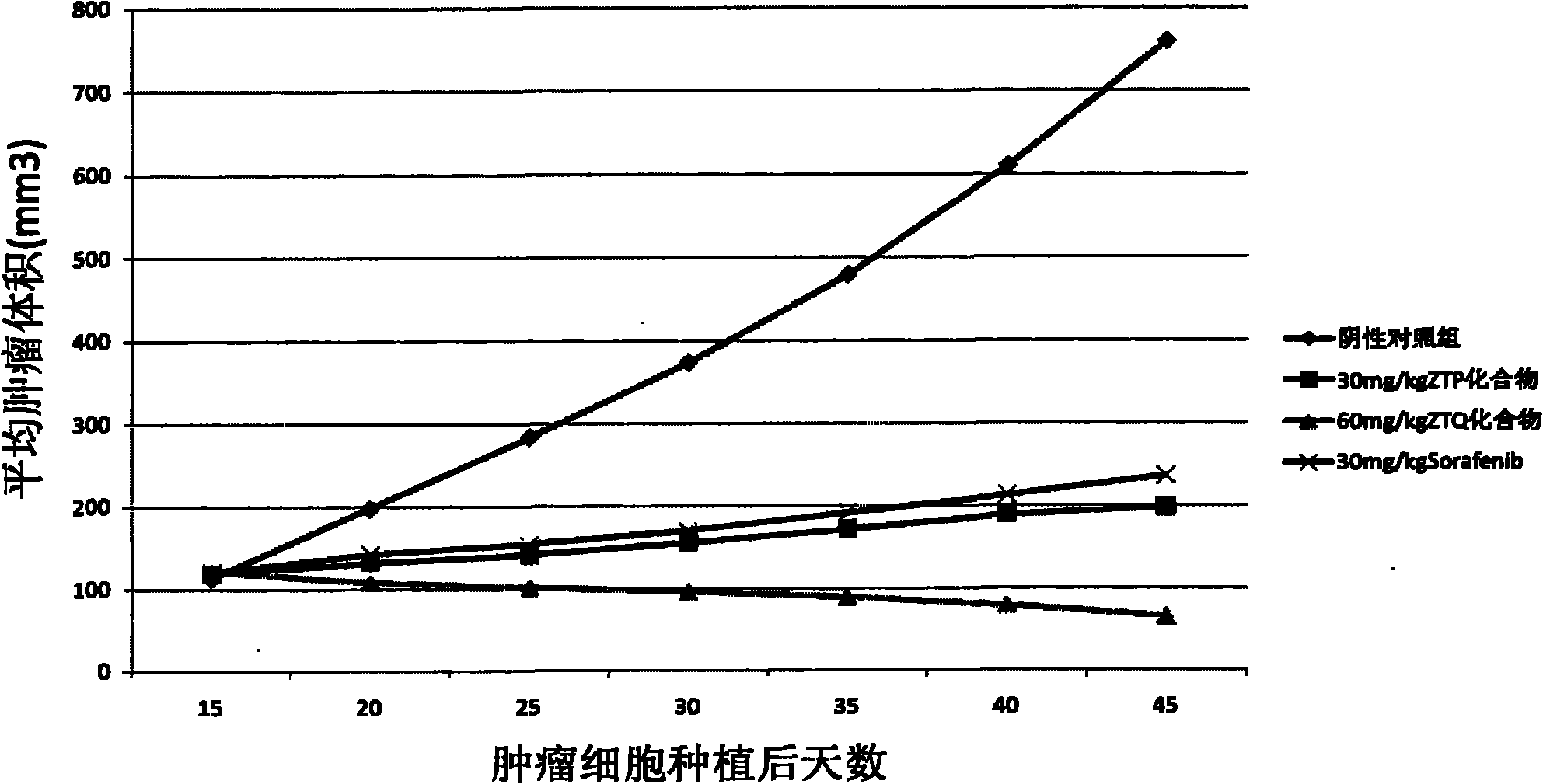
Anticancer compound and preparation method thereof
A compound, phenyl technology, applied in the field of anti-cancer compounds, can solve the problems of non-compliant interventional therapy, poor liver function, and low curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] (1) Preparation of N-[4-chloro-3-(trifluoromethyl)phenyl]-[4-(N-methyl-formamide)(4-pyridyloxy)phenyl]-thiourea
[0025] The specific operation is as follows:
[0026] 1. Preparation of 4-chloropyridine-2-carbonyl chloride (ZTP-1):
[0027] At 40°C, add 2 mL of dimethylformamide dropwise to 20 g (0.16 mol) of pyridine-2-carboxylic acid in 80 mL of thionyl chloride solution, after the addition is complete, stir at this temperature for 10 minutes, then raise the temperature to to 72°C, stirred overnight, LC-MS (liquid chromatography-mass spectrometer) showed that the reaction was not complete, added 20 mL of thionyl chloride, and continued to react at 72°C for 3 hours, LC-MS showed no change in the reaction, cooled to room temperature, remove thionyl chloride under reduced pressure, add 200 mL of toluene, evaporate to dryness under reduced pressure, then add 30 mL of toluene, and the solution is directly used for the next reaction;
[0028] 2. Preparation of 4-chloro(2-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com