Process for producing concentrated and purified heparin
A production process, heparin technology, applied in the production process of concentrated and purified heparin, can solve the problems of low extraction rate of active ingredients, many operation steps, and large resin damage, and achieve the effects of shortening the production cycle, high investment efficiency, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
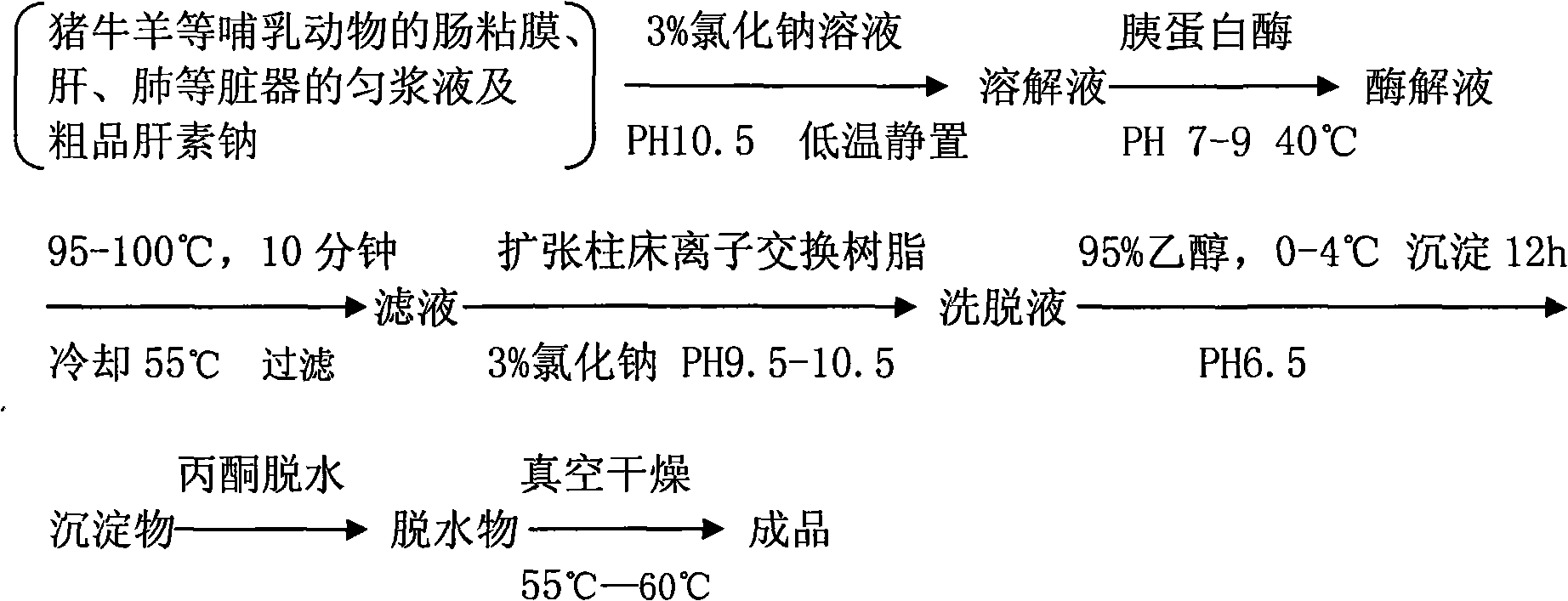
[0031] a) Add about 50 g of water to 250 L of tissue homogenate of small intestinal mucosa of mammals such as pigs, cattle, sheep, etc., add NaCl to 3%, stir, stand still, and filter.
[0032] b) Add 0.25% trypsin to the filtrate, enzymatically hydrolyze at pH 8.5, 40°C for several hours, adjust the pH to 9.0 with NaOH, raise the temperature to 50°C, keep it warm for 2h, keep stirring, then quickly heat up to 92-95°C, keep for 10- After 15 minutes, filter with an 80-mesh filter while it is still hot, and use the filtrate (I) for later use.
[0033] c) Take an appropriate amount of strong alkaline D254 dry resin, soak it fully in distilled water, swell it and filter it dry, add an equal volume of ethanol or acetone and stir for 1 hour, wash it with distilled water and filter it dry, add 4 times the amount of 2mol / l hydrochloric acid solution Stir for 2 hours, wash with distilled water until neutral, filter dry; add 2 times the amount of 2mol / l sodium hydroxide solution and stir...
Embodiment 2
[0041] a) Dissolve 10 g of crude heparin in 100 ml of 3% sodium chloride solution, then adjust the pH to 9 with 2% sodium hydroxide solution, let stand at 18° C. for 6 hours, vacuum filter, and use the filtrate for later use.
[0042] b) Add 0.03% trypsin to the above liquid, enzymolyze at pH 8.5, 40°C for 4 hours, heat up to 98-100°C for 10 minutes, vacuum filter while hot, and filtrate (I) for later use.
[0043] c) Take an appropriate amount of strong alkaline D254 dry resin, soak it fully in distilled water, swell it and filter it dry, add an equal volume of ethanol or acetone and stir for 1 hour, wash it with distilled water and filter it dry, add 4 times the amount of 2mol / l hydrochloric acid solution Stir for 2 hours, wash with distilled water until neutral, filter dry; add 2 times the amount of 2mol / l sodium hydroxide solution and stir for 2 hours, wash with distilled water until neutral, filter dry; finally use 2 times the amount of 2mol / l hydrochloric acid solution S...
Embodiment 3
[0051] a) Add about 30 g of water to 250 L of tissue homogenate of liver, lung and other organs of mammals such as pigs, cattle, sheep, etc., add NaCl to 5%, stir, stand still, and filter.
[0052] b) Add 0.4% papain to the above liquid, enzymolyze at pH 8.5, 40°C for 4 hours, heat up to 98-100°C for 10 minutes, vacuum filter while hot, and filtrate (I) for later use.
[0053] c) Take an appropriate amount of strong alkaline D254 dry resin, soak it fully in distilled water, swell it and filter it dry, add an equal volume of ethanol or acetone and stir for 1 hour, wash it with distilled water and filter it dry, add 4 times the amount of 2mol / l hydrochloric acid solution Stir for 2 hours, wash with distilled water until neutral, filter dry; add 2 times the amount of 2mol / l sodium hydroxide solution and stir for 2 hours, wash with distilled water until neutral, filter dry; finally use 2 times the amount of 2mol / l hydrochloric acid solution Stir for 2 hours, wash with water until ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com