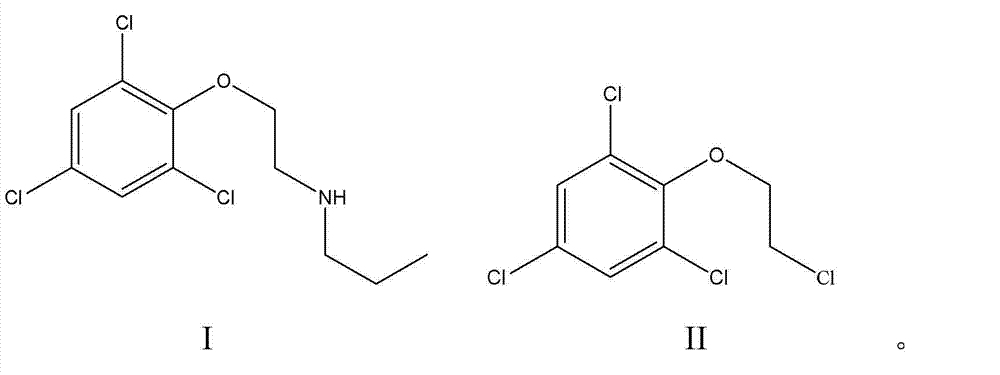
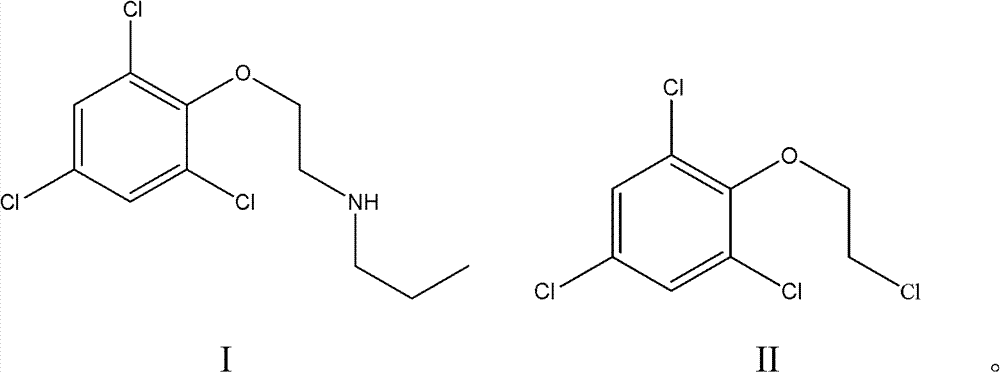
Synthesis method for prochloraz intermediate
A synthesis method and intermediate technology are applied in the field of prochloraz intermediate N-[2-ethyl]-N-n-propylamine, which can solve the problems of long amination reaction cycle, shortened reaction time, low yield and the like, Achieve the effect of eliminating vacuum distillation operation, shortening reaction time and saving cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Take 26g of 2-(2,4,6-trichlorophenoxy)chloroethane in a 250mL three-necked flask, add 25mL of n-propylamine, 100mL of ethanol and 0.85g of catalyst Pd / γ-Al 2 o 3 (loading capacity 1%), reflux at 80 ° C, TLC tracking reaction, 8h reaction is complete, after the reaction ends, filter, get filter cake and filtrate, filter cake is soaked 24 hours with dehydrated alcohol, obtains the catalyst Pd / γ of recovery -Al 2 o 3 0.80g, the filtrate was decolorized with activated carbon, cooled to room temperature and then filtered, and the filtrate was evaporated to remove the solvent under reduced pressure to obtain the product N-[2-(2,4,6-trichlorophenoxy)ethyl]-N-n-propylamine 27.6g, yield 96%, purity 99% (detected by liquid chromatography).
Embodiment 2
[0035] Take 26g of 2-(2,4,6-trichlorophenoxy)chloroethane in a 250mL three-necked flask, add 50mL of n-propylamine, 130mL of ethanol and 0.78g of catalyst Pd / γ-Al 2 o 3 (Loading capacity 1%), reflux at 80 ℃, TLC tracking reaction, 8h reaction is complete, after the reaction finishes, filter, get filter cake and filtrate, filter cake soaks 48 hours with dehydrated alcohol, obtains the catalyst Pd / γ- Al 2 o 3 0.75g, the filtrate was decolorized with activated carbon, cooled to room temperature and then filtered, and the filtrate was evaporated to remove the solvent under reduced pressure to obtain the product N-[2-(2,4,6-trichlorophenoxy)ethyl]-N-n-propylamine 27.5g, yield 96%, purity 99.5% (detected by liquid chromatography).
Embodiment 3
[0037] Take 26g of 2-(2,4,6-trichlorophenoxy)chloroethane in a 250mL three-necked flask, add 15mL of n-propylamine, 52mL of ethanol and 1.3g of catalyst Pd / γ-Al 2 o 3 (loading capacity 2%), reflux under 80 ℃, TLC tracking reaction, 10h reaction is complete, after reaction finishes, filter, obtain filter cake and filtrate, filter cake soaks 48 hours with dehydrated alcohol, obtains the catalyst Pd / γ- Al 2 o 3 1.25g, the filtrate was decolorized with activated carbon, cooled to room temperature and then filtered, and the filtrate was evaporated to remove the solvent under reduced pressure to obtain the product N-[2-(2,4,6-trichlorophenoxy)ethyl]-N-n-propylamine 26.1g, yield 92%, purity 99.2% (detected by liquid chromatography).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com