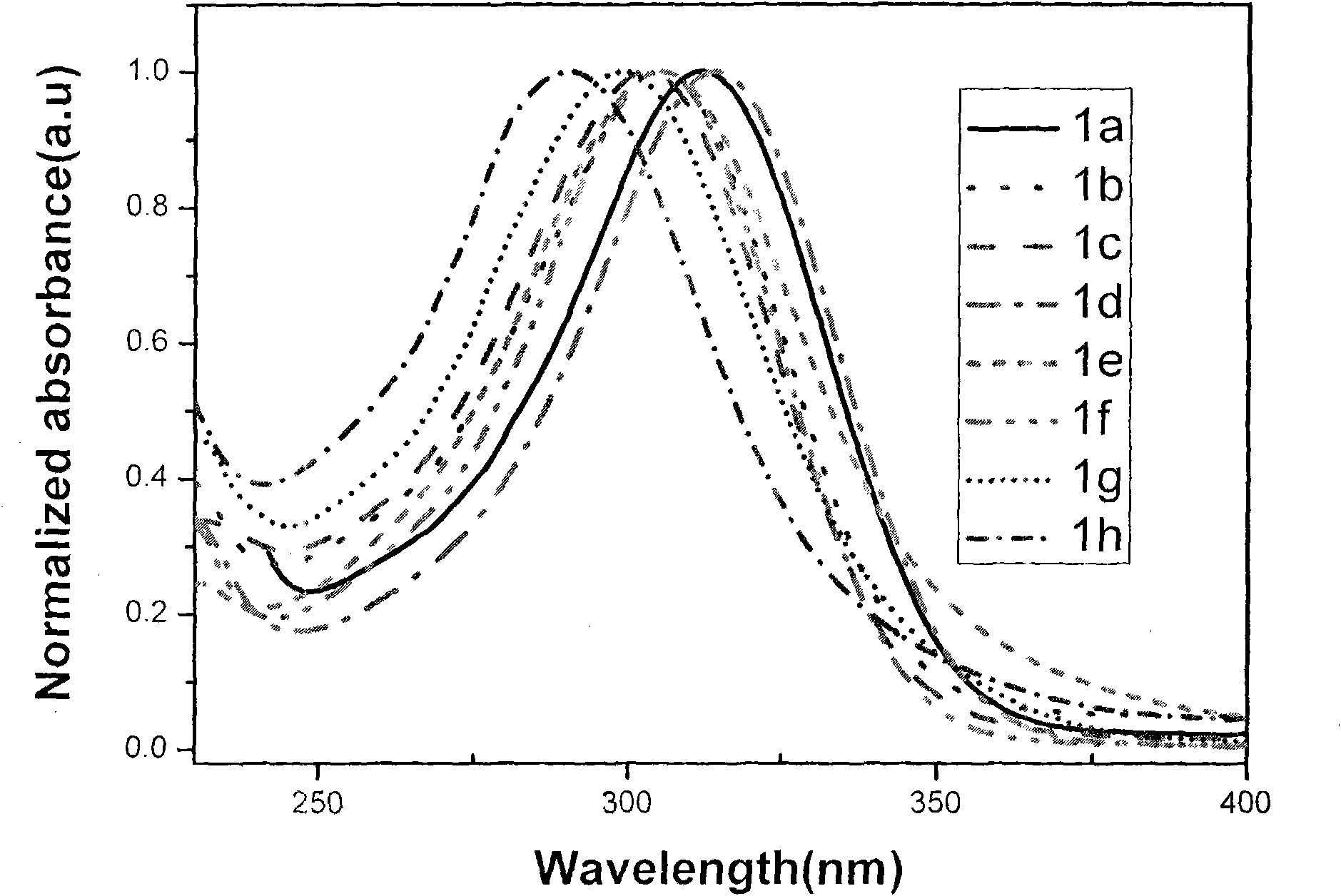
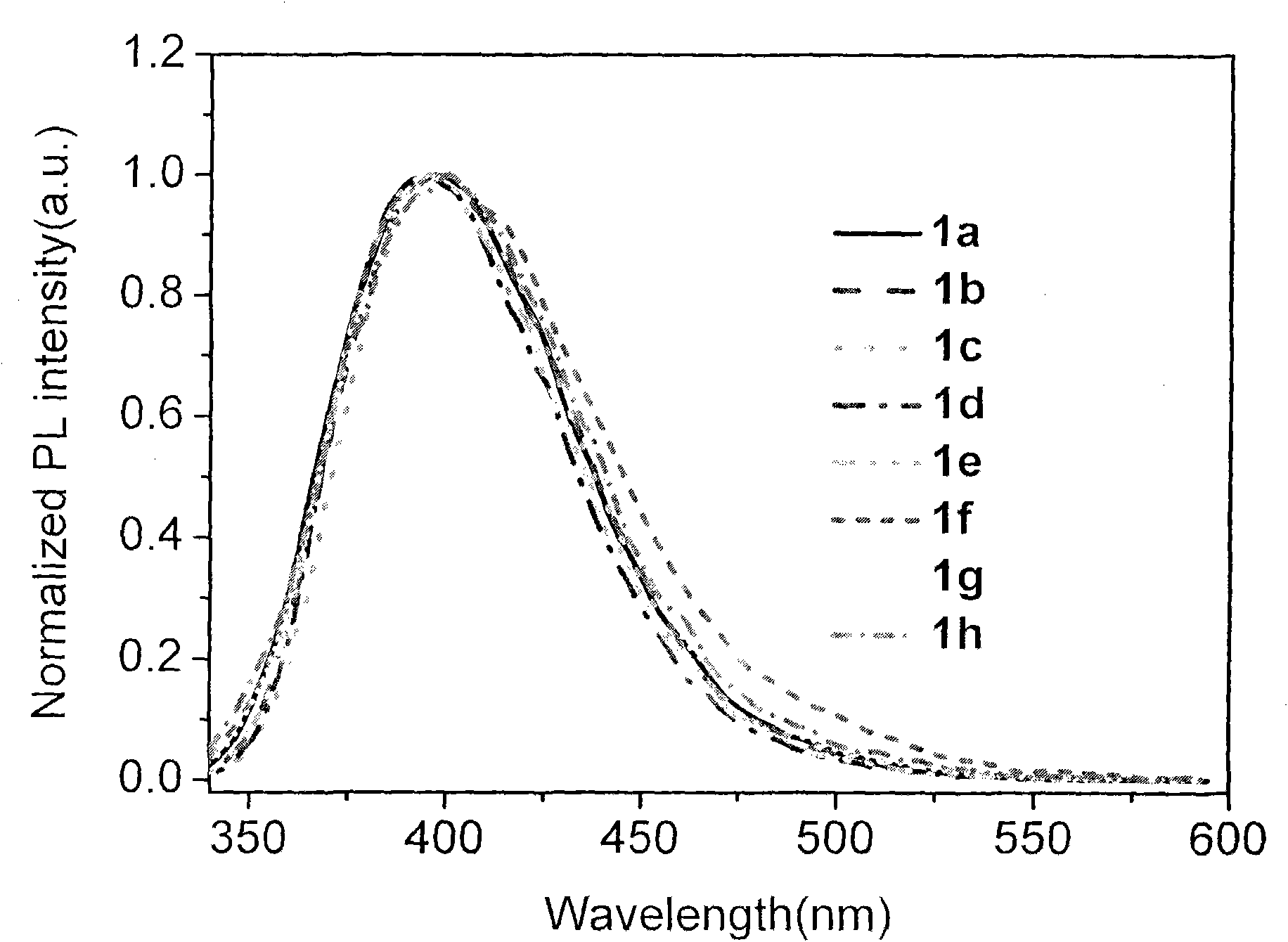
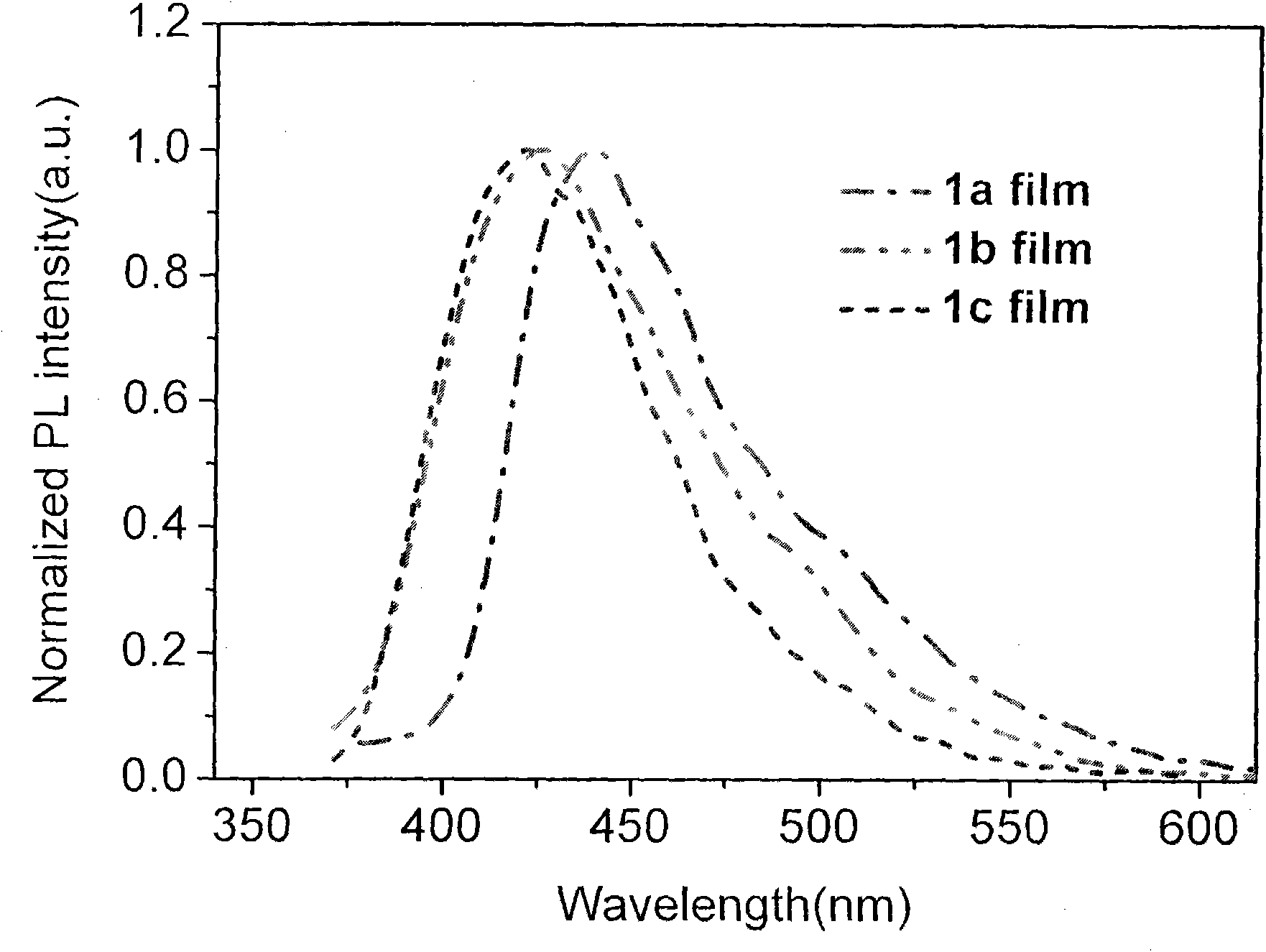
Terphenyl bridged-bis-benzimidazole quaternary ammonium compound, synthetic method and application thereof
A bisbenzimidazole and terphenyl bridge technology, applied in the field of optoelectronic materials, can solve problems such as increased manufacturing cost, poor solubility, and inability to use cost spin coating film-forming methods, and achieves improved application performance, improved solubility, and improved Effect of Carrier Transport Ability and Fluorescence Quantum Efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: [1,1'; 4',1"]-terphenyl-4,4"-bis(1,3-dimethyl-2-benzimidazolium ammonium bromide) 1a
[0031] In a 100mL autoclave with magnetic stirring, put 2.31g (5mmol) of 4,4"-bis-benzimidazolyl-p-terphenyl, 40ml of dimethylformamide, 1.06g (10mmol) of anhydrous sodium carbonate, and 4.72g of methyl bromide (50mmol), close the autoclave, heat up to 80-100°C, react at this temperature for 12 hours, cool to 60°C, vacuum out excess methyl bromide and solvent dimethylformamide, add 50mL chloroform to the kettle to dissolve , filtered out the insoluble matter, concentrated chloroform under reduced pressure to obtain a yellow crude product, and the crude product was subjected to column chromatography (dichloromethane / methanol 8:1) to obtain 2.85 g of a pure product with a yield of 83.7% and a melting point greater than 350°C. 1 H NMR (DMSO-d 6 , 300MHz): δ8.25-8.21(m, 8H), 8.06(d, 8H), 7.81-7.78(m, 4H), 4.09(S, 12H). 13 C NMR (DMSO-d 6 , 300MHz): δ149.68, 143.15, 138.37, 1...
Embodiment 2
[0032] Example 2: [1,1'; 4',1"]-terphenyl-4,4"-bis(1,3-diethyl-2-benzimidazolium bromide) 1b
[0033] In a 100mL autoclave with magnetic stirring, put 2.31g (5mmol) of 4,4"-bis-benzimidazolyl-p-terphenyl, 40mL of dimethylformamide, 1.38g (10mmol) of anhydrous potassium carbonate, bromoethane 3.27g (30mmol), in a closed autoclave, heat up to 110-125°C, react at this temperature for 18 hours, cool to 70°C, vacuum out excess bromoethane and solvent dimethylformamide, and pour into the autoclave Add 50 mL of chloroform to dissolve, filter out the insoluble matter, concentrate the chloroform under reduced pressure to obtain a pale yellow crude product, and recrystallize from acetonitrile to obtain 2.8 g of the pure product, with a yield of 75.6%. The melting point is 267-269°C. 1 H NMR (DMSO-d 6 , 300MHz): δ8.25-8.21(m, 8H), 8.06(d, 8H), 7.80-7.77(m, 4H), 4.36(q, 8H), 1.40(t, 12H). 13 C NMR (DMSO-d 6 , 300MHz): δ149.59, 143.25, 138.40, 130.82, 130.80, 127.78, 127.61, 126.59, 120...
Embodiment 3
[0034] Example 3: [1,1'; 4',1"]-terphenyl-4,4"-bis(1,3-dibutyl-2-benzimidazolium ammonium bromide) 1c
[0035] In a 100mL autoclave with magnetic stirring, put 2.31g (5mmol) of 4,4"-bis-benzimidazolyl-p-terphenyl, 50mL of methanol, 1.06g (10mmol) of anhydrous sodium carbonate, and 5.48g of 1-bromobutane (40mmol), close the autoclave, heat up to 100-120°C, react at this temperature for 10 hours, cool to 60°C, vacuum out excess 1-bromobutane and solvent methanol, add 50mL chloroform to the kettle to dissolve , filtered out insoluble matter, and concentrated chloroform under reduced pressure to obtain a yellow crude product, which was separated by column chromatography to obtain 2.6 g of pure product, with a yield of 61.3% and a melting point of 304-306°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com