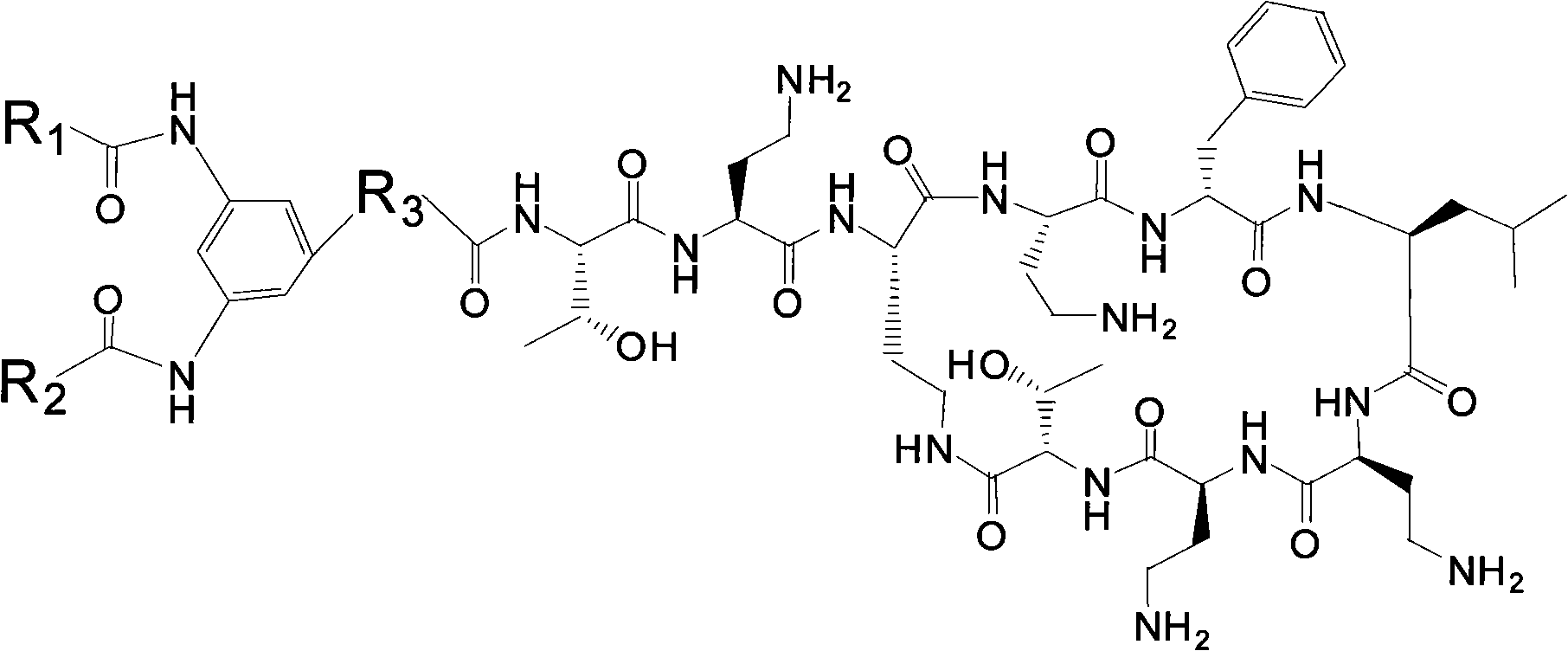
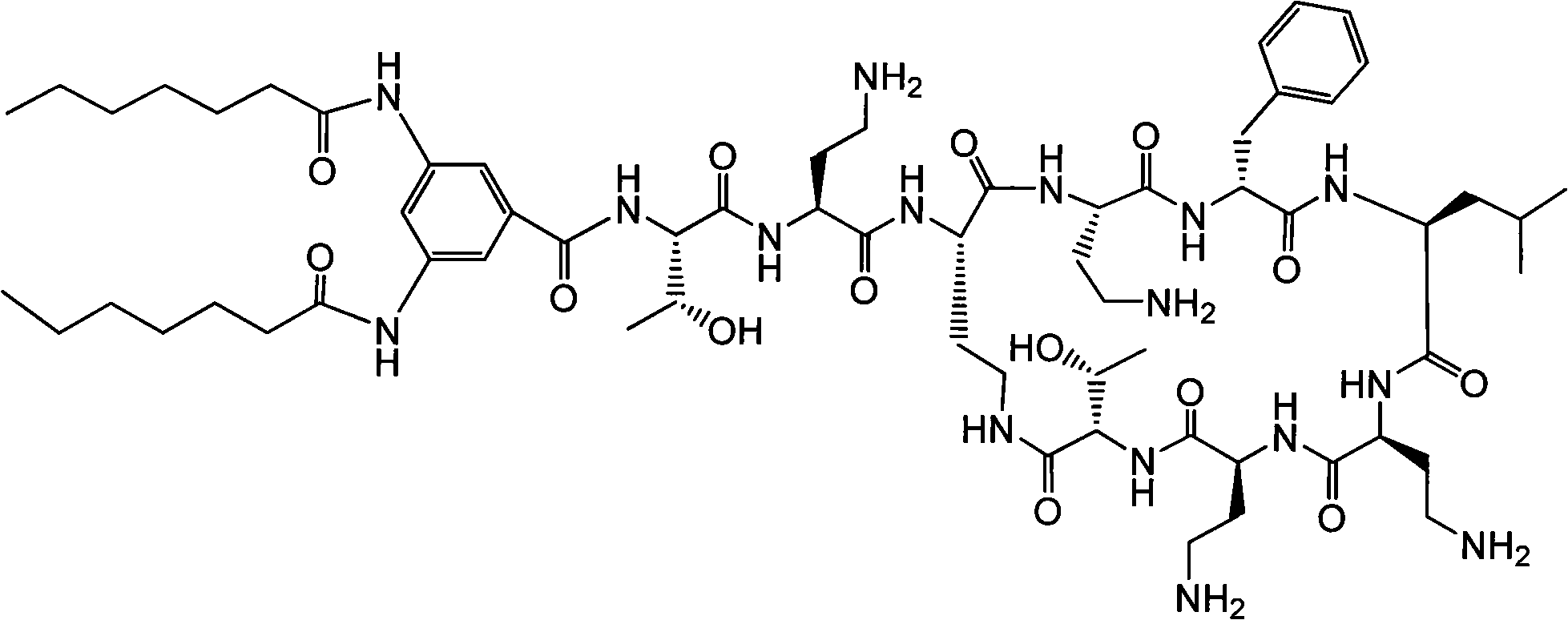
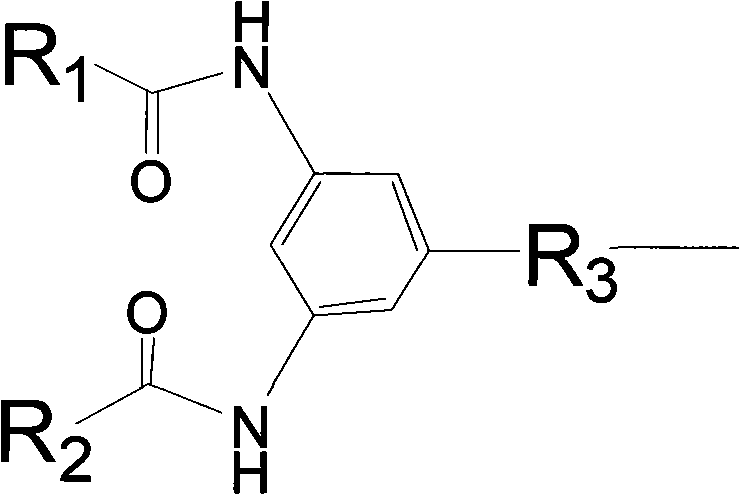
Polymyxin derivative and preparation method thereof
A technology of polymyxin and derivatives, applied in the field of polymyxin derivatives and its preparation, can solve the problems of nephrotoxicity and neurotoxicity, limited application, lack of treatment methods, etc., and achieve reduced physiological toxicity and high antibacterial activity , Improve the effect of antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: Carry out enzymatic hydrolysis to polymyxin B, the steps are as follows:
[0051] (1) Dissolve 35 grams of polymyxin B sulfate in 3 liters of 0.07M phosphate buffer (pH 7.0);
[0052] (2) Dissolve 6,000 U of ficin in 0.5 liter of 0.07M phosphate buffer (pH 7.0), and mix it with the above polymyxin B sulfate solution;
[0053] (3) Add 1 gram of dithiothreitol;
[0054] (4) After gentle mixing at 37°C for 24 hours, heat under reflux for a short time to inactivate the enzyme;
[0055] (5) After cooling, filter to remove the denatured enzyme, and adjust the pH to 2.0 with 6M HCl;
[0056] (6) Extract four times with 0.25 liter of n-butanol, and adjust the pH of the aqueous phase to 8.5 with 6M NaOH;
[0057] (7) Extract with n-butanol again, and adjust the pH of the aqueous phase to 5.1 with 6M HCl;
[0058] (8) The aqueous phase was concentrated under reduced pressure to 0.2 liters, and desalted using a Sephadex G-10 desalting column;
[0059] (9) After ...
Embodiment 2
[0061] Embodiment 2: to the chemical modification of enzymolysis product, the steps are as follows:
[0062] (1) The polymyxin B product after the above enzymatic hydrolysis was prepared into a 0.05M solution with water: dioxane: triethylamine (1:1:1, v / v), and 5M 2- (tert-butoxycarbonyloxyimino)-2-phenylacetonitrile;
[0063] (2) Incubate at 23°C for 20 minutes, add excess methanolic ammonia solution to terminate the reaction;
[0064] (3) Concentrate the reaction solution by rotary evaporation to dryness, redissolve with methanol, and filter;
[0065] (4) excessive diethyl ether is added in the filtrate, and the precipitation is collected by filtration;
[0066] (5) Purify the precipitate using flash silica gel column chromatography, the mobile phase is triethylamine:methanol:dichloromethane (1:13:86, v / v);
[0067] (6) Collect the main elution peak, and obtain the intermediate compound after freeze-drying;
[0068] (7) The intermediate compound is mixed with excess fluo...
Embodiment 3
[0072] Embodiment 3: to the chemical modification of enzymolysis product, step (1) to step (6) are the same as embodiment 2, and step (7) is as follows:
[0073] (7) The intermediate compound is mixed with excess fluorenylmethoxycarbonyl (Fmoc)-3,4-diaminophenylacetic acid-pentafluorophenyl ester, and condensed in the presence of dimethylformamide;
[0074] Step (8) to step (10) are the same as embodiment 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com