Non-noble metal fuel cell oxygen reduction electrocatalyst
A fuel cell and electrocatalyst technology, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of catalyst catalytic activity and stability gap, high preparation cost, expensive and other problems, which is conducive to large-scale production and reduces preparation Cost and manpower saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] This embodiment uses cobalt chloride (CoCl 2 ·6H 2 O), melamine, acetylene black prepare fuel cell oxygen reduction electrocatalyst of the present invention, and this catalyst is represented by CoMe / C, and wherein Me represents that nitrogen source is melamine, and C represents acetylene black.
[0019] (1) Acetylene black pretreatment: Acetylene black is treated with 1.5M HNO before use 3 Stir for 10 hours, then wash and dry for later use.
[0020] (2) Cobalt chloride (CoCl 2 ·6H 2 O), melamine, and acetylene black were ground in an agate mortar for 30 minutes to make the precursors uniformly mixed.
[0021] (3) Divide the precursor mixture into three equal parts, put them into three ceramic boats, and then transfer the ceramic boats to the middle of the tubular resistance furnace.
[0022] (4) The tubular resistance furnace is fed into N 2 protective gas.
[0023] (5) Heat treatment at 600°C for 2 hours: use step-by-step heating, keep at 100°C, 200°C, 300°C, 40...
Embodiment 2
[0028] According to the method step of embodiment 1, the Vulcan XC-72R carbon black of U.S. Cabot Company is used to replace acetylene black to prepare catalyst, all the other conditions are unchanged, the gained catalyst is marked as CoMe / V, and " V " represents VulcanXC-72R carbon black.
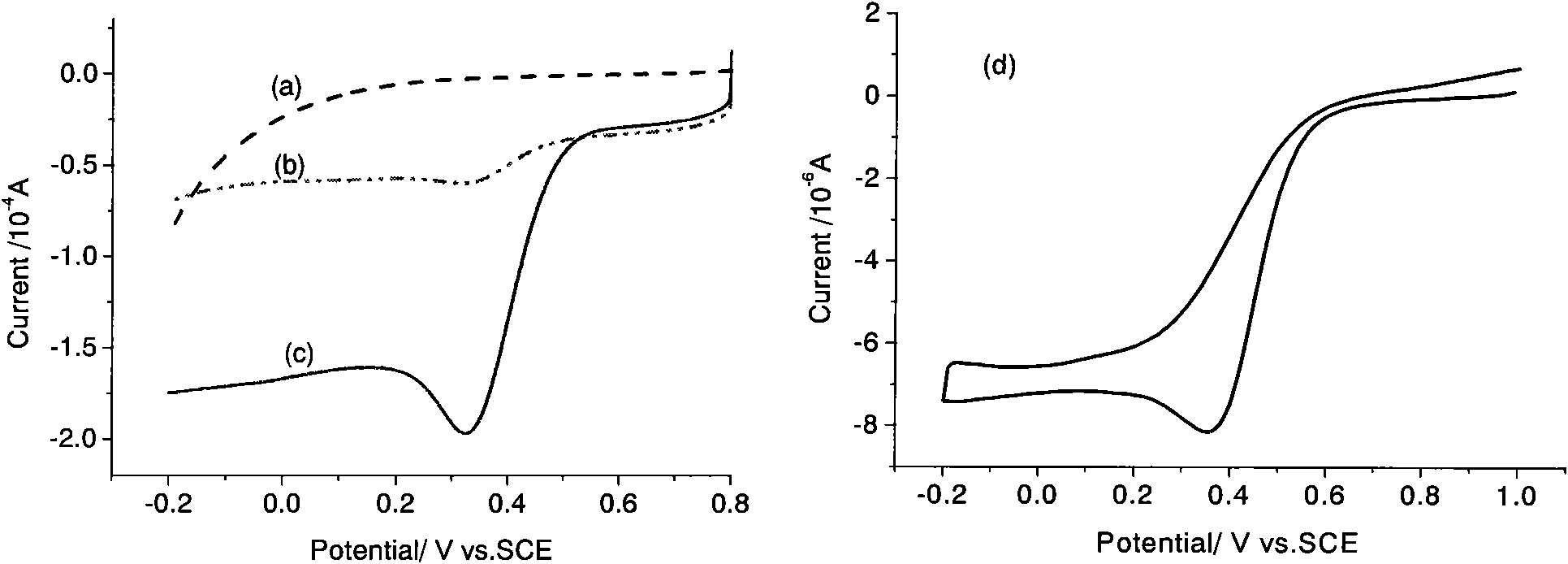
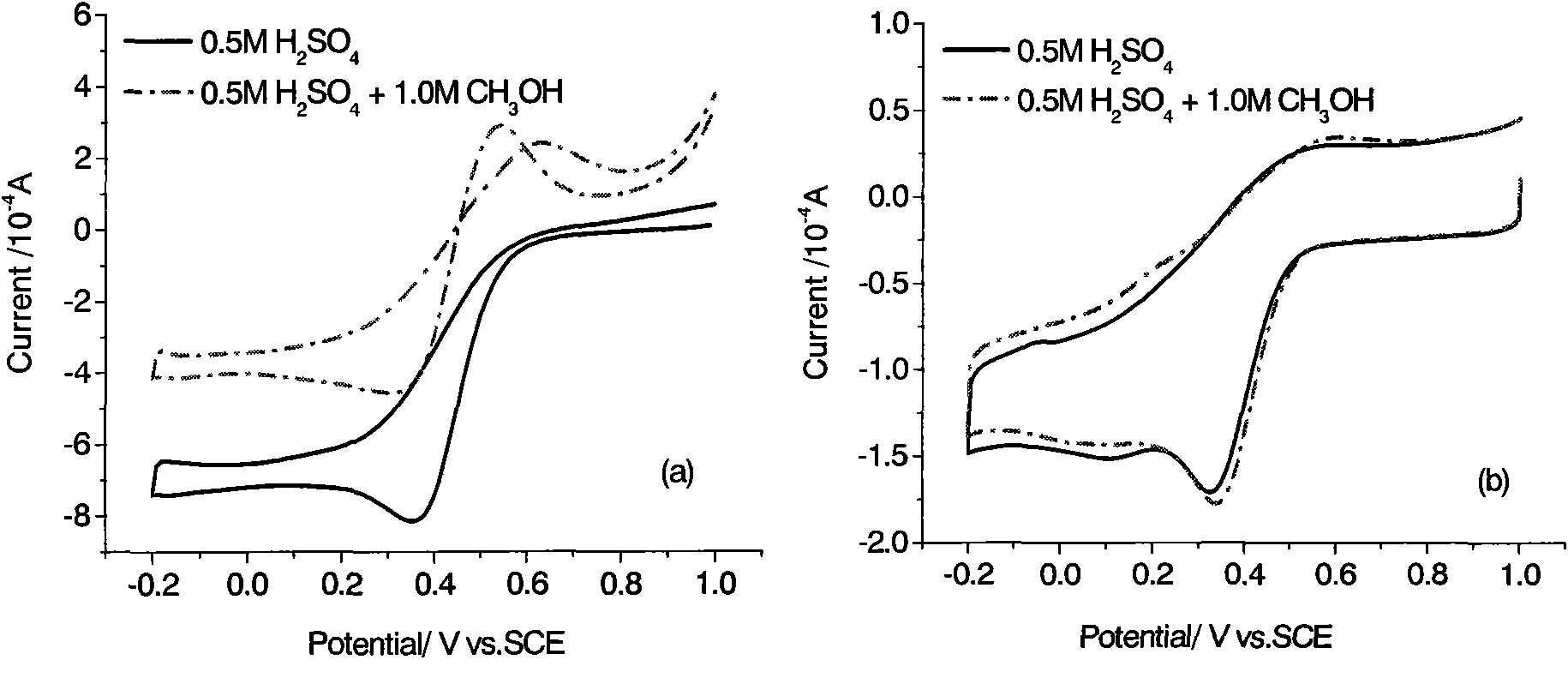
[0029] Figure 4 It is the fuel cell oxygen reduction catalyst CoMe / V that embodiment 2 obtains at 0.5M H 2 SO 4Electrocatalytic activity in solution. The scanning speed is 5mV / s, and the test temperature is 30°C. It can be seen that the oxygen reduction catalysts obtained with two different carbon blacks have similar catalytic activities. Therefore, it is of practical value to use acetylene black as a raw material for preparing catalysts.
Embodiment 3
[0031] According to the method steps of Example 1, an equivalent amount of hexamethylenetetramine was used instead of melamine to prepare the catalyst, and the other conditions were unchanged. The obtained catalyst was marked as CoHMTA / C, and "HMTA" means hexamethylenetetramine.
[0032] Figure 5 It is the fuel cell oxygen reduction catalyst CoHMTA / C that embodiment 3 obtains at 0.5M H 2 SO 4 Electrocatalytic activity in solution. The scanning speed is 5mV / s, and the test temperature is 30°C. It can be seen that CoHMTA / C also has good catalytic activity for oxygen reduction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com