Structure and application of target programmed cell death protein 5 (PDCD5) anti-influenza virus oligonucleotide
An oligonucleotide, influenza A virus technology, applied in the field of bioengineering drugs, to achieve the effects of less toxic and side effects, important social and economic benefits, and high specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
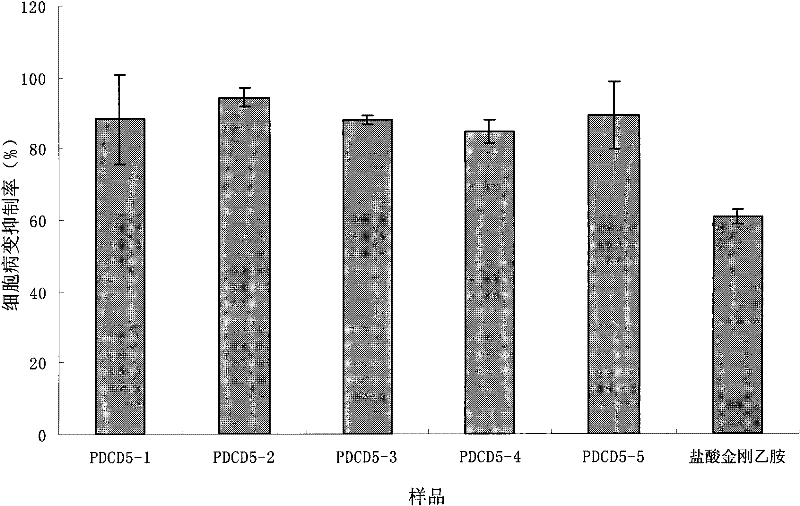
[0034] This example mainly illustrates the design, synthesis and screening of anti-influenza virus antisense oligonucleotides targeting human PDCD5 gene.
[0035] Materials and Methods
[0036] 1. Design and synthesis of antisense oligonucleotide PDCD5-5
[0037]Retrieve the nucleic acid sequence database in GeneBank, select the PDCD5 mRNA reference sequence NM_004708.2 published by NCBI, and perform computer simulation of the RNA secondary structure, and select 5 unstable stem-loop structures as antisense oligonucleotides target. Through online blast sequence comparison with GeneBank, the selected target sequences have good specificity and will not interfere with the expression of other normal human genes (see Table 1). All oligonucleotides were synthesized with 8909 type automatic DNA synthesizer to synthesize antisense oligonucleotides modified with full sulfur. After the synthesis was completed, the concentrated ammonia solution was cleaved and deprotected at 55°C for 1...
Embodiment 2
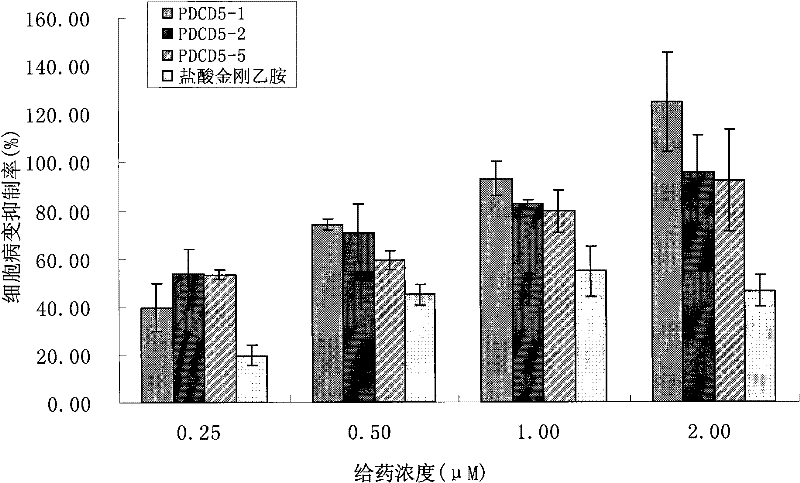
[0057] This example mainly illustrates the anti-influenza virus effect of antisense oligonucleotides PDCD5-5 with different lengths.
[0058] 1. Optimization of PDCD5-5 sequence length one
[0059] Referring to the mRNA sequence of the human PDCD5 gene, the PDCD5-5 sequence was increased or decreased by bases at both ends, so that the sequence was 22, 18, 16, and 14 bases. Through online blast sequence comparison with GeneBank, the selected target sequences have good specificity and will not interfere with the expression of other normal human genes. Referring to Example 1, oligonucleotide synthesis, sulfur modification, purification, drying and storage were performed.
[0060] Referring to Example 1, A549 cells were plated on a 96-well cell culture plate, and 60-80% of them were confluent the next day. Dilute PDCD5-5 to 0.25, 0.5, 1.0, 2.0 μM with 1640 containing 1% FBS. The cells were washed once with PBS (pH7.5), and different concentrations of PDCD5-5 with a length of 22...
Embodiment 3
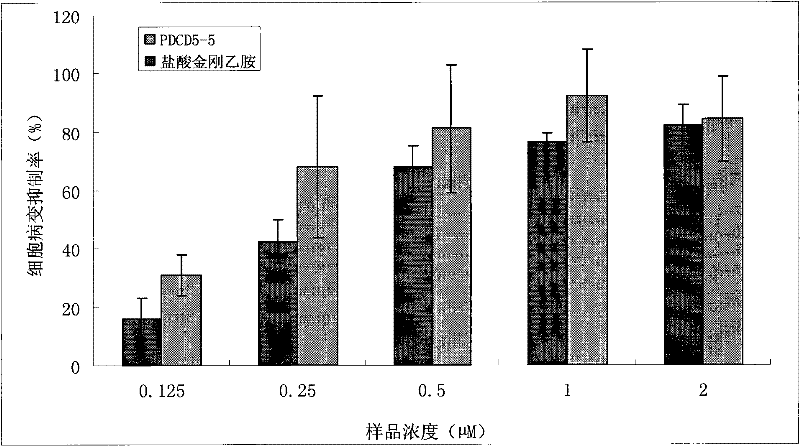
[0072] This example mainly illustrates the research on the inhibition of H1N1 cytopathic activity by rimantadine-modified PDCD5-5 at the level of A549 cells in vitro.
[0073] Materials and Methods
[0074] 1. Coupling of rimantadine to PDCD5-5
[0075] After dehydrochlorination, rimantadine hydrochloride exposes the amino group, and undergoes a nucleophilic substitution reaction with 6-maleimide caproic acid N-succinimide ester to replace the succinimide ester to form a new compound. Re-react with antisense oligonucleotides modified with sulfhydryl groups at 5', and finally form the target rimantadine-antisense oligonucleotide coupling compound. The coupling process is as follows: Figure 10 . Use the optimized synthetic route and the reaction conditions of each step to synthesize, synthesize the rimantadine modified sequence (PJG) of PDCD5-5 coupled with high-purity rimantadine and PDCD5-5, and use various analytical methods to ensure the obtained coupling The compound ha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com