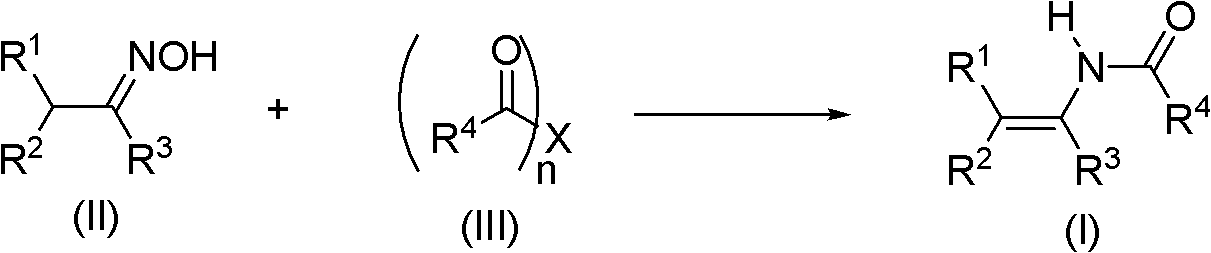Method for synthesizing acrylamide compound
An enamide and compound technology, applied in the field of organic synthesis, can solve the problems of harsh reaction conditions, high toxicity, air sensitivity, etc., and achieve the effects of mild reaction conditions, low production cost, and high synthesis yield
Inactive Publication Date: 2010-11-03
NORTHWEST UNIV(CN)
View PDF3 Cites 6 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, the existing technology for synthesizing enamides has disadvantages such as harsh reaction conditions, high cost of catalysts or reducing agents, limited range of substrates, and low yields, which are not suitable for large-scale production.
Patents WO 99 / 18065, M.Burk (J.Org.Chem., 1998, 63, 6084) and X.Zhang (J.Org.Chem., 1999, 64, 1775) describe iron powder / acetic anhydride / acetic acid Reduction system, reducing acylated ketoxime to synthesize enamide compounds, but the chemical amount of iron powder will cause local overheating in the reaction system, resulting in unsmooth reaction and low yield
Chinese patent application CN200480038602.0 describes the preparation of enamide compounds by hydrogen and acid anhydride reduction acylation ketoxime in the presence of noble metals (iridium, rhodium, etc.), but the use of a large amount of noble metals makes this reaction cost too high
Chinese patent application CN200780013686.6 describes the preparation of enamide compounds by reductive acylation of alkylphosphine and acid anhydride, but alkylphosphine compounds have disadvantages such as air sensitivity, high toxicity, and harsh reaction conditions.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
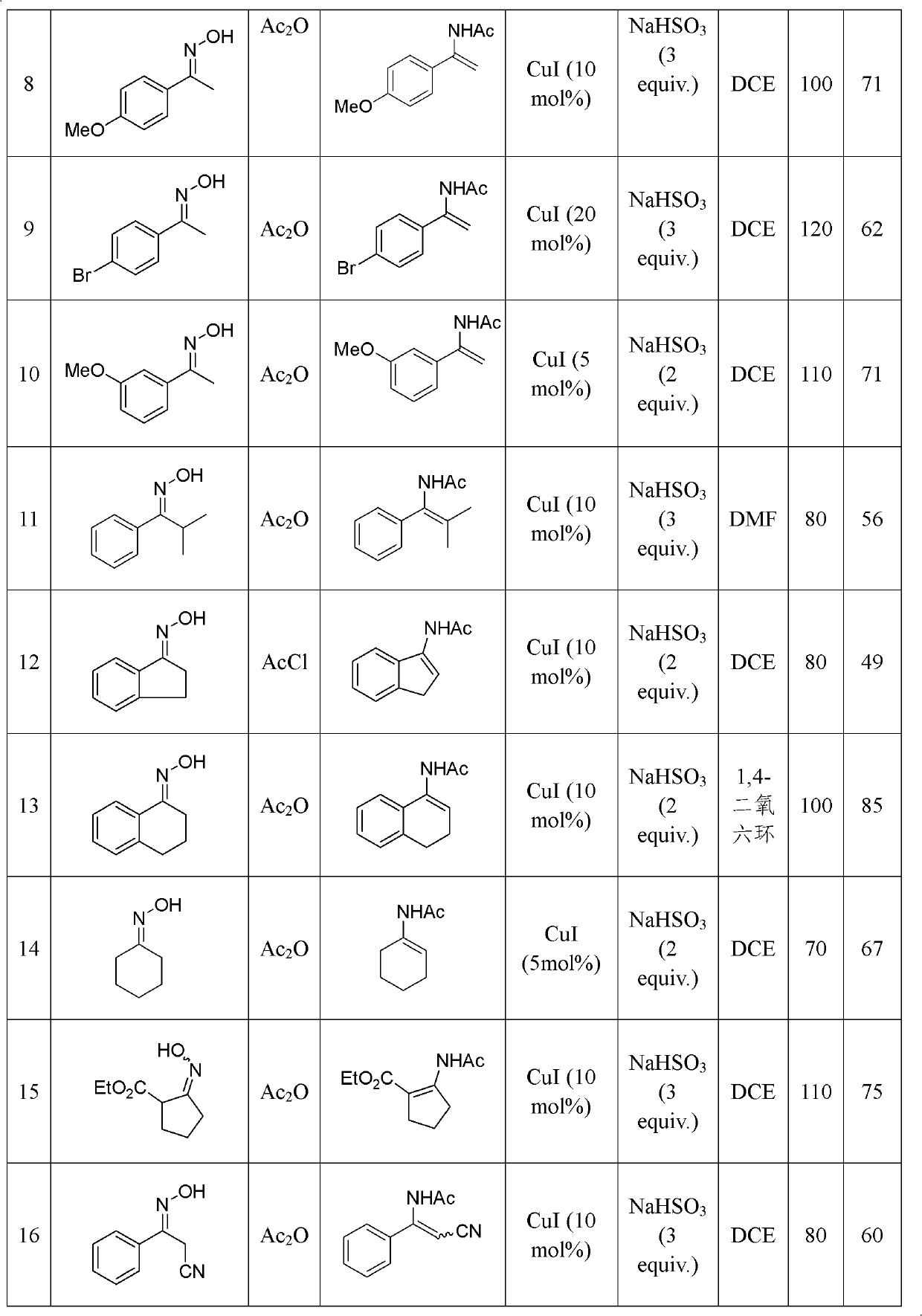
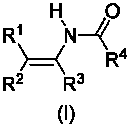
The invention discloses a method for synthesizing an acrylamide derivative as shown in the synthesis formula (I). The acrylamide derivative is obtained by performing reductive acylation and isomerization on an oxime derivative and an acyl compound in the presence of a Pd, Cu or Ni transition metal catalyst and a proper reducing agent, wherein R1, R2 and R3 independently are hydrogen atoms, aryl, aryloxy, alkyl, cyclalkyl, alkoxy, heterocyclyl, carboxyl, esteryl, cyano, sulfonyl and carbamoyl; or R1 and R2 are combined into ring; or R2 and R3 are combined into a ring; R4 is hydrogen atom, alkyl or aryl; n is 1 or 2; and when n is 1, X is a leaving group and when n is 2, X is an oxygen atom. A series of high-yield substituted acrylamide compounds can be obtained under a mild condition by reducing and acylating oxime in the presence of the transition metal catalyst. The method has the characteristics of mild reaction condition, simple operation, wide application range, low production cost, high synthesis yield and the like.
Description
technical field The invention relates to a synthesis method of enamide compounds, in particular to a synthesis method suitable for large-scale production of enamide compounds, and belongs to the technical field of organic synthesis. Background technique In the modern pharmaceutical industry, enamide compounds are widely used in asymmetric or symmetrical hydrogenation reactions to prepare chiral or achiral amine compounds (T.C.Nugent, M.El-Shazly, Adv.Synth.Catal.2010, 352, 753 -819). In organic synthesis, enamides can also act as nucleophiles to participate in the formation of carbon-carbon bonds and carbon-nitrogen bonds (R. Matsubara, S. Kobayashi, Acc. Chem. Res. 2008, 41, 292-301). However, the existing technologies for synthesizing enamide compounds have disadvantages such as harsh reaction conditions, high cost of catalysts or reducing agents, limited range of substrates, and low yields, which are not suitable for large-scale production. Patents WO 99 / 18065, M.Burk ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07B43/06C07C231/10C07C233/05C07C233/18C07C233/13C07C233/06C07C233/52C07C233/47C07C315/04C07C317/28C07D213/40C07D307/52C07D333/20
Inventor 关正辉任智卉张志远杨秉勤
Owner NORTHWEST UNIV(CN)
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com