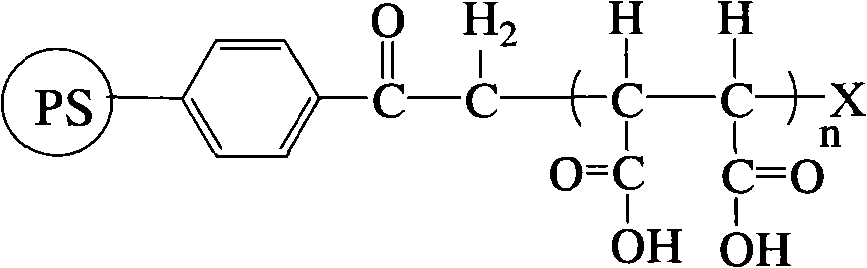
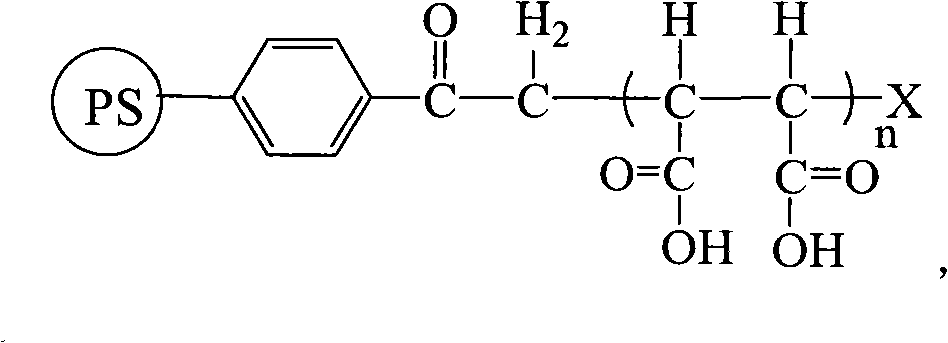
Polystyrene-graft-succinic carboxylic acid resin
A technology of butanedicarboxylic acid and polystyrene, applied in the field of functional polymers, can solve the problems of low loading of butanedicarboxylic acid, difficulty in obtaining PS resin, and affecting performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 0.1g PS-acyl-Cl resin (crosslinking degree: 7% divinylbenzene, particle size: 200μm) into a single-necked flask, add dimethyl sulfoxide, swell for 12h, add 6.4g maleic anhydride, 0.05g CuCl , 0.08g 2,2'-bipyridine, vacuuming and nitrogen filling were alternately carried out 5 times, and the reaction was carried out under magnetic stirring at 110°C for 24h under nitrogen atmosphere. A PS resin grafted with maleic anhydride in a loading amount of 6.6 mmol / g was obtained.
[0026] Take 0.1 g of the above resin in a single-necked flask, add 10 mL of 0.5 mol / L NaOH solution, hydrolyze at 40 °C for 10 h, filter with a sand core, soak in 0.8 mol / L hydrochloric acid solution and wash it with deionized water several times until The washing liquid was neutral, and the supported amount was 12.1 mmol / g of carboxylic acid resin and 6.0 mmol / g of butanedicarboxylic acid resin.
Embodiment 2
[0028] Add 0.1g PS-acyl-Cl resin (crosslinking degree: 85% divinylbenzene, particle size: 1000μm) into a single-necked flask, add dimethyl sulfoxide, swell for 8h, add 7.2g maleic anhydride, 0.05g CuCl , 0.2mL of pentamethyldiethylenetriamine, evacuated and filled with nitrogen alternately for 5 times, and reacted with magnetic stirring at 80°C for 20h under nitrogen atmosphere. A PS resin grafted with maleic anhydride in a loading amount of 5.7 mmol / g was obtained.
[0029] Take 0.1 g of the above resin in a single-necked flask, add 10 mL of 1.5 mol / L NaOH solution, hydrolyze for 3 hours at 55 ° C, filter with a sand core, soak in 2 mol / L hydrochloric acid solution, and then wash it with deionized water for several times until clean The liquid was neutral, and a carboxylic acid resin with a supported amount of 10.4 mmol / g was obtained.
Embodiment 3
[0031] Add 0.1g PS-acyl-Br resin (crosslinking degree: 7% divinylbenzene, particle size: 10μm) into a single-necked flask, add N,N'-dimethylformamide, swell for 10h, add 12.6g Malay Acid anhydride, 0.07g CuBr, 0.2mL tetramethylethylenediamine, vacuumize and nitrogen-filled alternately 5 times, and react under magnetic stirring at 110°C for 6h under nitrogen atmosphere. A PS resin grafted with maleic anhydride in a loading amount of 7.8 mmol / g was obtained.
[0032] Take 0.1 g of the above resin in a single-necked flask, add 10 mL of 0.2 mol / L NaOH solution, hydrolyze at 80°C for 5 hours, filter with a sand core, soak in 0.5 mol / L hydrochloric acid solution and wash it with deionized water for several times until The cleaning liquid was neutral, and the carboxylic acid resin with a supported amount of 14.3 mmol / g was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com