Preparation method of Oxiracetam
A molar weight and hydroxyl technology, applied in the field of medicinal chemistry, can solve the problems of many reaction steps, complicated operations, and many impurities, and achieve the effects of low cost, simple operation and high product yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
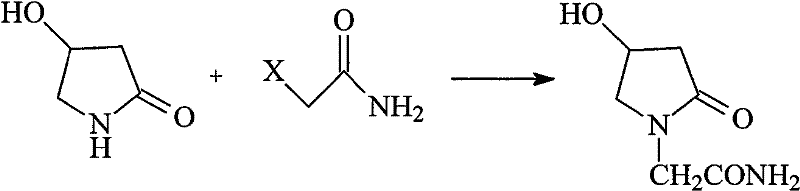
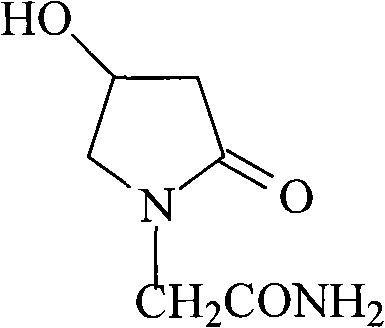
[0032] In a 500ml four-neck flask equipped with stirring, thermometer, reflux condenser and drying tube, add 90ml of methanol, slowly add 9.4g of sodium metal in portions, stir and dissolve at room temperature, then add 40.4g (0.4mol) of 4-hydroxyl -2-Pyrrolidone, heated to reflux. Stir and reflux for 1 hour, distill methanol off, add 100ml of dry toluene, continue distilling until the toluene is completely evaporated, and cool to room temperature. Add 100ml of dimethyl sulfoxide, 4.6g, 0.02mol of benzyltriethylammonium chloride (TEBA), drop 41.2g, 0.4mol of 2-chloroacetamide at 30°C, and dissolve it in 200ml In the dimethyl sulfoxide solution, after the dropwise addition was completed, the temperature was raised to 80° C., and the reaction was carried out for 2 hours. After the reaction was completed, filter the filtrate to remove the solvent under reduced pressure, and recrystallize 100 ml of the residue with isopropanol to obtain 18.5 g of white crystals. Tested by YRT-3 ...
Embodiment 2
[0034] In a 500ml four-neck flask equipped with stirring, thermometer, reflux condenser and drying tube, add 90ml of methanol, slowly add 9.4g of sodium metal in portions, stir and dissolve at room temperature, then add 40.4g (0.4mol) of 4-hydroxyl -2-Pyrrolidone, heated to reflux. Stir and reflux for 1 hour, evaporate methanol, add 100ml of dry toluene, continue distillation, wait until the toluene is evaporated, and cool to room temperature. Add 100ml of N,N-dimethylformamide, 4.6g, 0.02mol of benzyltriethylammonium chloride (TEBA), and drop 55.2g, 0.4mol of 2-bromoacetamide at 30°C to make It was dissolved in 200ml of N,N-dimethylformamide solution, and after the dropwise addition was completed, the temperature was raised to 80°C and reacted for 2h. After the reaction was completed, filter the filtrate to remove the solvent under reduced pressure, and recrystallize the residue with 100 ml of isopropanol to obtain 18 g of white crystals. Tested by YRT-3 melting point appar...
Embodiment 3
[0036] In a 500ml four-neck flask equipped with stirring, thermometer, reflux condenser and drying tube, add 90ml of methanol, slowly add 9.4g of sodium metal in portions, stir and dissolve at room temperature, then add 40.4g (0.4mol) of 4-hydroxyl -2-Pyrrolidone, heated to reflux. , stirred and refluxed for 1h, evaporated methanol, added dry toluene (100ml), continued distillation, until the toluene was evaporated, cooled to room temperature. Add 100ml of dry toluene, 6.4g, 0.02mol of tetrabutylammonium bromide (TBAB), and dropwise add 41.2g, 0.4mol of 2-chloroacetamide at 50°C to dissolve it in a solution of 200ml of toluene. After the addition was completed, the temperature was raised to 100° C., and the reaction was carried out for 2 hours. After the reaction was finished, it was filtered, and the filtrate was evaporated to remove the solvent under reduced pressure, and the residue was recrystallized with 100ml of isopropanol to obtain 13g of white crystals. Tested by YR...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com