Piperacillin-sulbactam sodium medicinal composition liposome injection
A technology of piperacillin sodium sulbactam sodium and cillin sodium sulbactam sodium, which is applied in the field of medicine and can solve the problems of poor stability of sodium sulbactam sodium, unfull appearance of freeze-dried powder injection, and failure to meet the requirements of pharmacy. , to achieve the effect of reducing the risk of medication, the process is practical, and it is conducive to mass production and promotion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
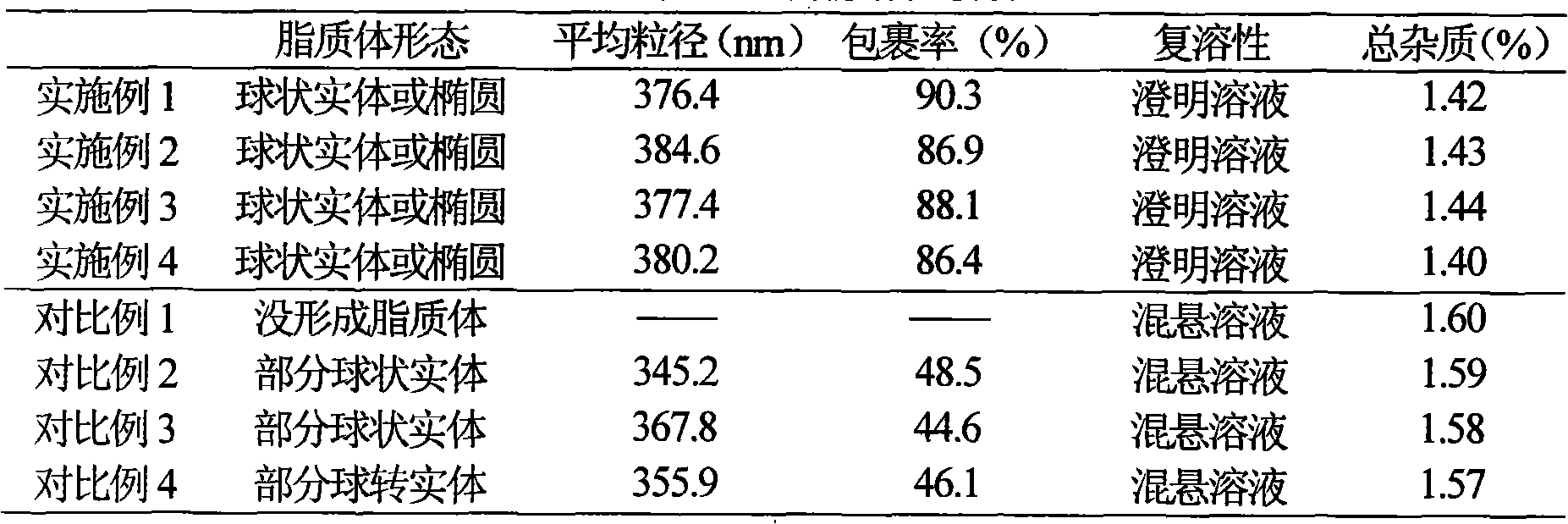
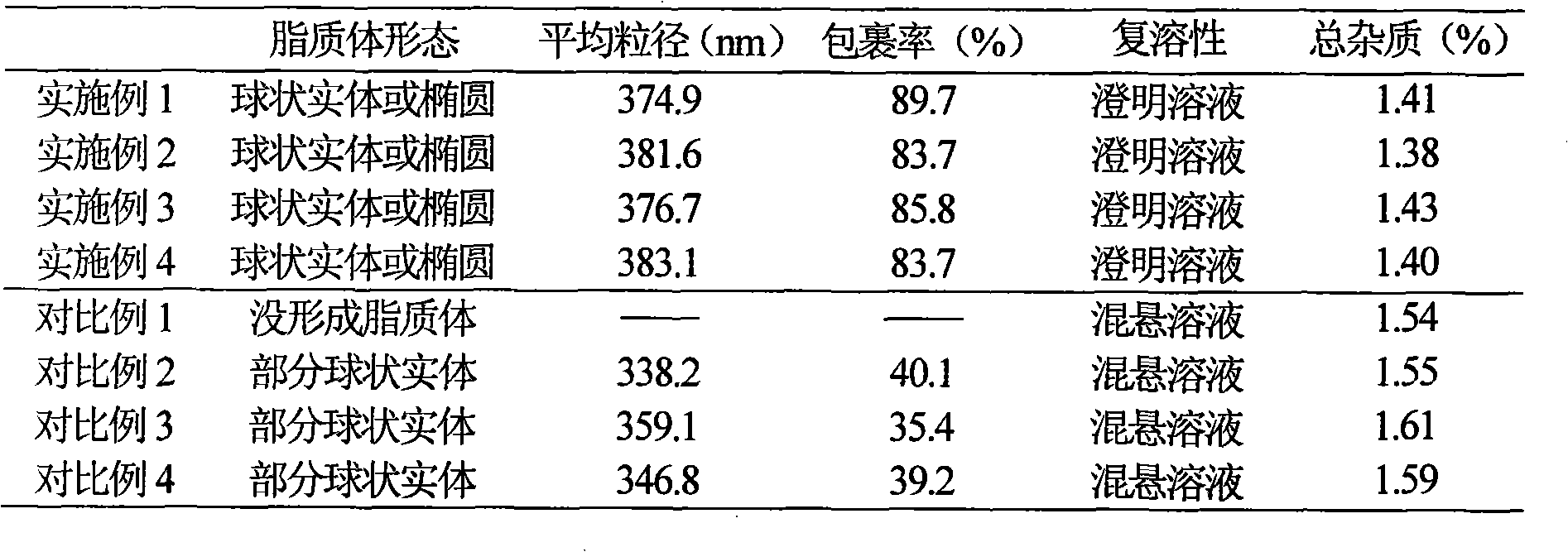
Embodiment 1
[0029] Example 1 Preparation of Piperacillin Sodium Sulbactam Sodium Liposome Injection
[0030] Prescription (100 bottles)
[0031] Piperacillin Sodium 200g
[0032] Sulbactam Sodium 50g
[0033] Sitosterol 187.5g
[0034] Octadecylamine 62.5g
[0035] Mannitol 16.6g
[0036] Lactose 8.3g
[0037] Preparation steps:
[0038] (1) Dissolving 187.5g sitosterol and 62.5g octadecylamine in 500ml ethanol, adding a pH value of 6.5 sodium dihydrogen phosphate solution, stirring and mixing the two solutions, and evaporating to remove the organic solvent;
[0039] (2) Dissolve 200g of piperacillin sodium, 50g of sulbactam sodium, 16.6g of mannitol, and 8.3g of lactose in the above solution, stir evenly and ultrasonically treat for 30min, then place it in a high-speed tissue masher (10000rpm) and rotate for 25min , to obtain liposome mother solution;
[0040] (3) The mother liquor is divided into vials, half-pressed, and placed in a freeze dryer for freeze-drying:
[0041] a. P...
Embodiment 2
[0044] Example 2 Preparation of Piperacillin Sodium Sulbactam Sodium Liposome Injection
[0045] Prescription (100 bottles)
[0046] Piperacillin Sodium 100g
[0047] Sulbactam Sodium 50g
[0048] Sitosterol 57.7g
[0049] Phosphatidylethanolamine 92.3g
[0050]Ribosan 11.25g
[0051] Sodium alginate 3.75g
[0052] Preparation steps:
[0053] (1) Dissolving 57.7g sitosterol and 92.3g phosphatidylethanolamine in 400ml isopropanol, adding a phosphate solution with a pH value of 7.3, stirring and mixing the two solutions, and evaporating the organic solvent;
[0054] (2) Dissolve 100g of piperacillin sodium, 25g of sulbactam sodium, 11.25g of riboglycan, and 3.75g of sodium alginate in the above solution, stir evenly and sonicate for 30min, then place in a high-speed tissue masher (10000rpm) Rotate in medium for 20min to obtain liposome mother solution;
[0055] (3) The mother liquor is divided into vials, half-pressed, and placed in a freeze dryer for freeze-drying:
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com