Method for preparing cobalt-base catalyst used for partial oxidation of methane for preparing synthesis gas
A cobalt-based catalyst, gas-cobalt-based technology, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve the problems of complex preparation process and achieve selectivity Good, good catalyst repeatability, easy conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Catalyst preparation: Dissolve P123 (4.5g) in 100ml of absolute ethanol at room temperature, add 7.5ml of concentrated nitric acid, add 10.2g of aluminum isopropoxide under vigorous stirring, stir until aluminum isopropoxide is completely dissolved, add 0.7535gCo(NO 3 ) 2 ·6H 2 O, and then dried in an oven at 60°C for 2 days, and the obtained sample was calcined in a muffle furnace at 700°C for 1 hour to obtain Co-Al with a mass percentage of Co of 6%. 2 o 3 catalyst.
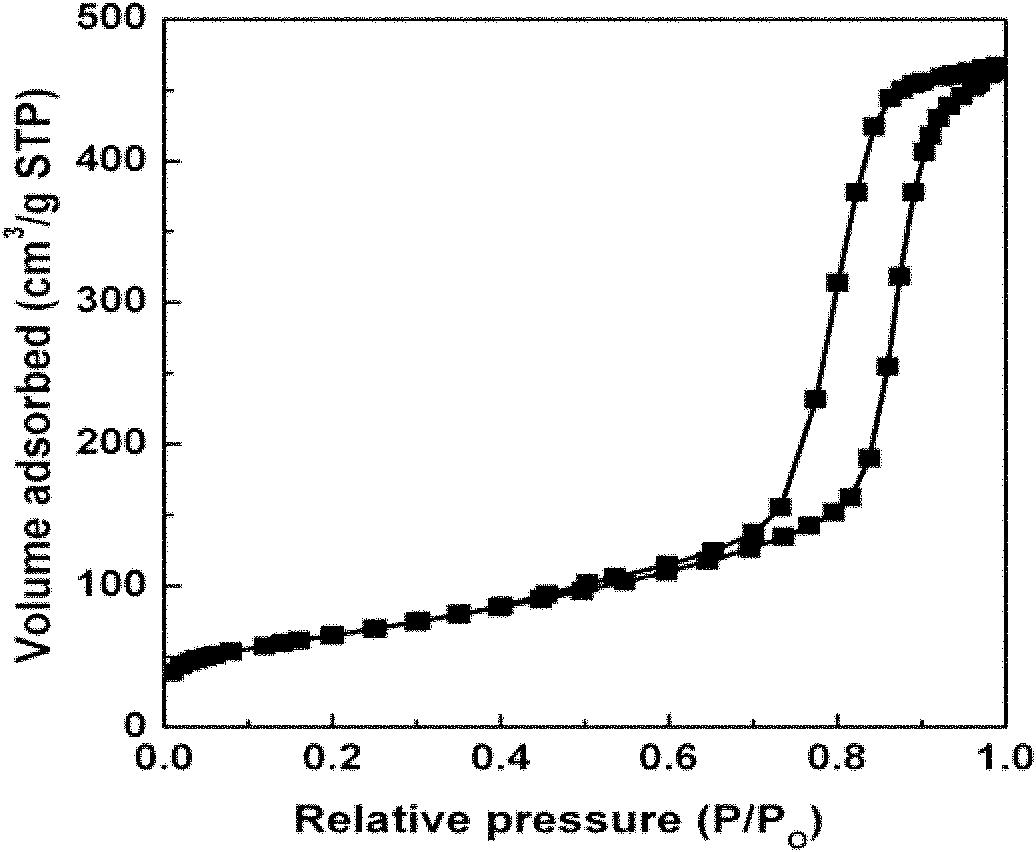
[0025] The high-magnification transmission electron microscope image of the prepared sample can be found in figure 1 , N of the prepared sample 2 Adsorption-desorption isotherms see figure 2 . Depend on figure 1 It shows that the catalyst has an ordered mesoporous structure, and no Co species agglomerated particles are found, indicating that the Co species is highly dispersed; in figure 2 There is an obvious type IV adsorption isotherm H1 hysteresis loop, indicating the existence of mesoporous...
Embodiment 2~4
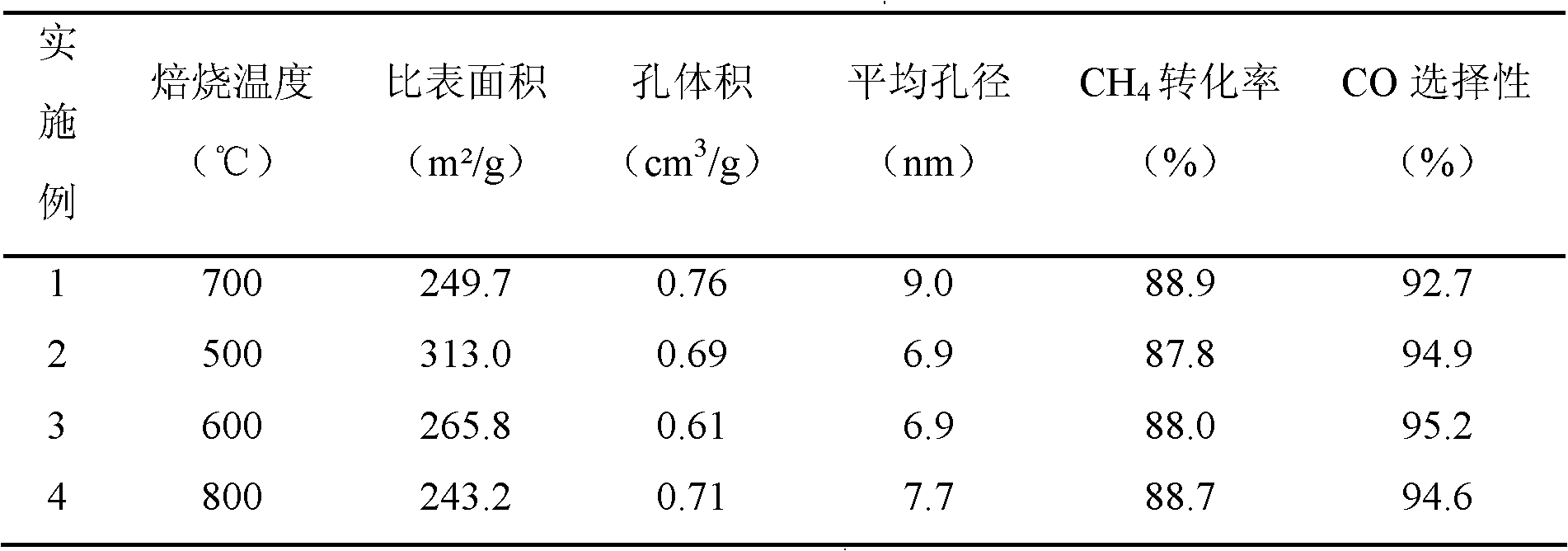
[0028] Catalyst preparation Referring to Example 1, the calcination temperature was changed to 500, 600 and 800°C respectively, and the rest of the conditions were the same. Catalysts prepared at different calcination temperatures were subjected to N 2 Low temperature physical adsorption characterization, the results are shown in Table 1. The evaluation conditions refer to Example 1, the reduction temperature is changed to 800°C, and the rest of the conditions are the same. The evaluation results are shown in Table 1.
[0029] Table 1 Co-Al prepared at different calcination temperatures 2 o 3 The specific surface area, pore structure and reaction performance of the catalyst
[0030]
Embodiment 5
[0032] Catalyst preparation with reference to embodiment 1, Co(NO 3 ) 2 ·6H 2 O is replaced by 0.646g Co(CH 3 CO 2 ) 2 4H 2 O, the rest of the conditions are the same, and Co-Al with a Co mass percentage of 6% is prepared 2 o 3 catalyst. The evaluation conditions are the same as in Example 1, and the evaluation results are shown in Table 2.
[0033] Table 2 Co-Al prepared by different precursor salts 2 o 3 Catalyst Reactivity
[0034]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com