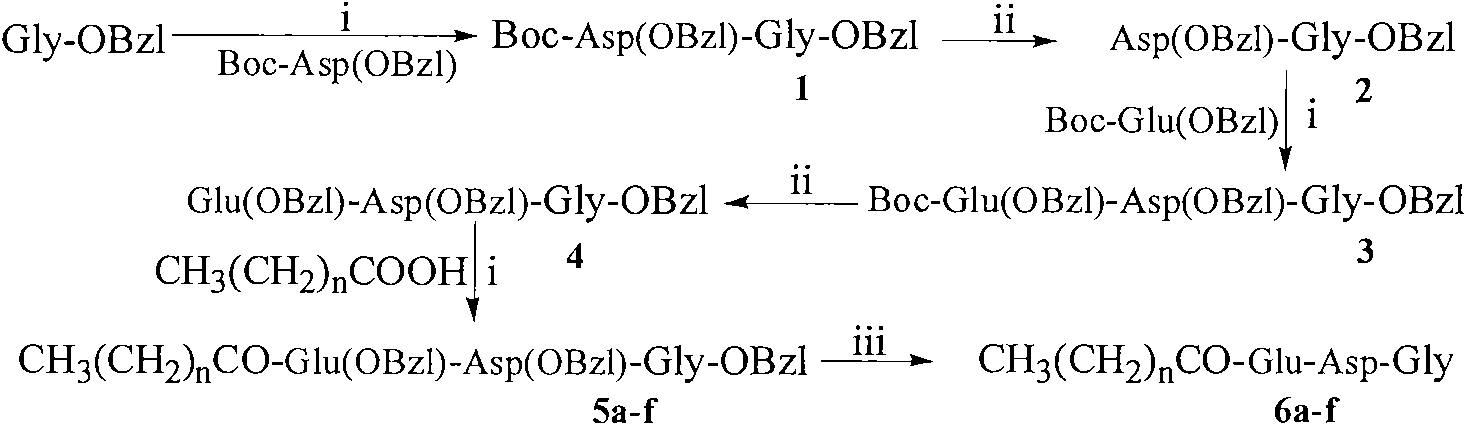Saturated fatty chain acid Glu-Asp-Gly tripeptide amide, synthetic method and application thereof
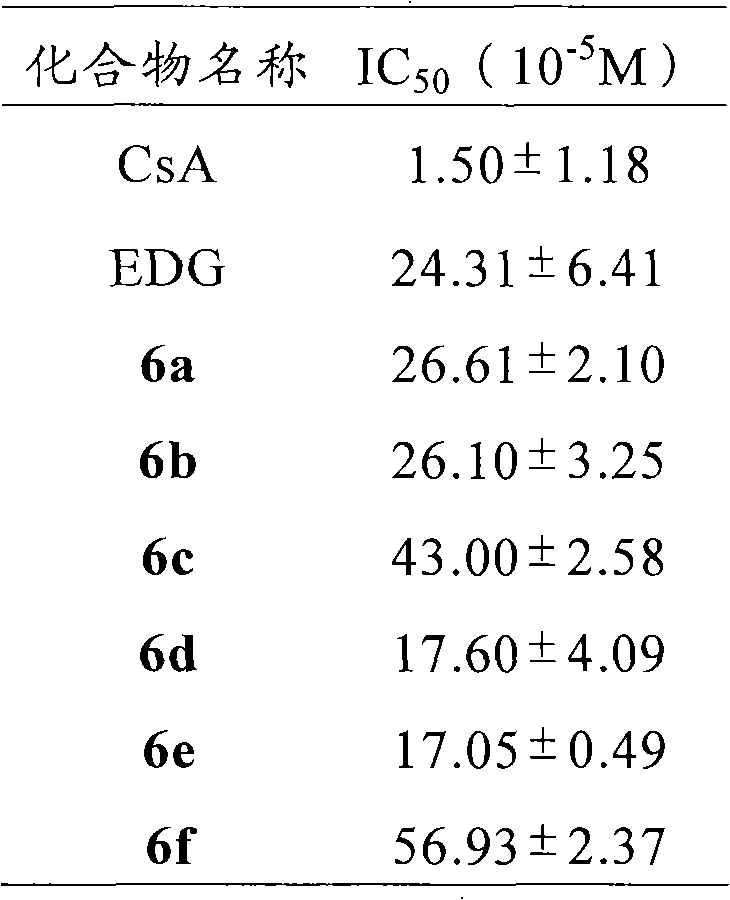
A technology of glu-asp-gly and fatty chain acid, which is applied in the direction of tripeptide components, peptide preparation methods, chemical instruments and methods, etc., can solve cyclosporine A's poor water solubility, dosage form or unsatisfactory curative effect, kidney disease, etc. Strong toxicity and other issues, to achieve excellent immunosuppressive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1B
[0028] The preparation of embodiment 1Boc-Asp(OBzl)-Gly-OBzl
[0029] 8.08g (25.00mmol) Boc-Asp (OBzl) was dissolved in 200ml anhydrous THF, and 3.38g (25.00mmol) N-hydroxybenzotriazole (HOBt) was added to the solution obtained under ice-cooling, and made completely dissolved. After 10 minutes, 6.20 g (30 mmol) of dicyclohexylcarbodiimide (DCC) was added to obtain the reaction solution (I), which was set aside. Suspend 8.43g (25.00mmol) TosH·Gly-OBzl in 20ml anhydrous THF under ice-cooling, then add 1ml N-methylmorpholine (NMM) to adjust the pH to 8-9. Stir for 35 minutes to obtain the reaction solution (II), which is ready for use. The reaction solution (I) was added to the reaction solution (II) under ice bath, stirred for 1 h under ice bath, and then stirred at room temperature for 12 h. TLC (chloroform / methanol, 10:1) showed that Boc-Asp(OBzl)-OH disappeared. Dicyclohexylurea (DCU) was filtered off and THF was removed under reduced pressure. The residue was dissolved w...
Embodiment 2
[0030] The preparation of embodiment 2HCl Asp (OBzl)-Gly-OBzl
[0031] Dissolve 11.04g (23.50mmol) Boc-Asp(OBzl)-Gly-OBzl in 250ml 4mol / l hydrogen chloride-ethyl acetate solution, stir at room temperature for 2 hours, TLC (chloroform:methanol, 5:1) shows that the raw material point disappears , concentrated under reduced pressure to remove ethyl acetate, and the residue was repeatedly added with a small amount of ether for concentration under reduced pressure to remove hydrogen chloride gas. Finally, a small amount of diethyl ether was added to triturate the residue to obtain 9.17 g (96%) of the title compound as a colorless solid powder, which was directly used in the next reaction. ESI-MS(m / z): 371[M+H] + .
Embodiment 3B
[0032] Embodiment 3 Preparation of Boc-Glu(OBzl)-Asp(OBzl)-Gly-OBzl
[0033] According to the method of Example 1, a beige oil was obtained from 7.61 g (22.56 mmol) Boc-Glu (OBzl) and 9.17 g (22.56 mmol) HCl·Asp (OBzl)-Gly-OBzl. The obtained compound was purified by silica gel column chromatography to obtain 10.73 g of a colorless oily product, the purification condition: chloroform:methanol=100:1, and the yield was 69%. ESI-MS(m / z): 690[M+H] + , [α] 20 D =-24.4 (c=1.0, CH 3 OH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com