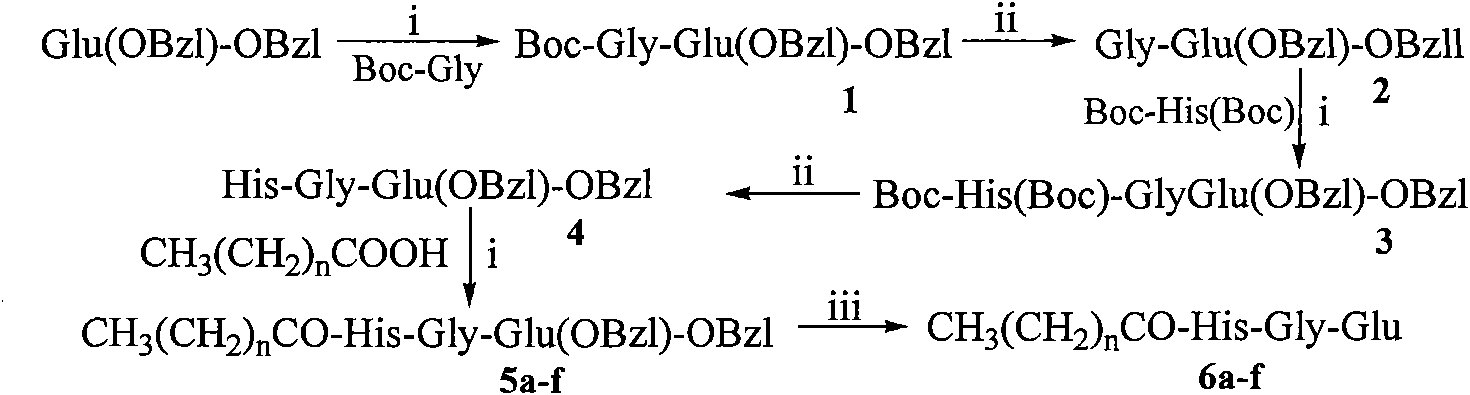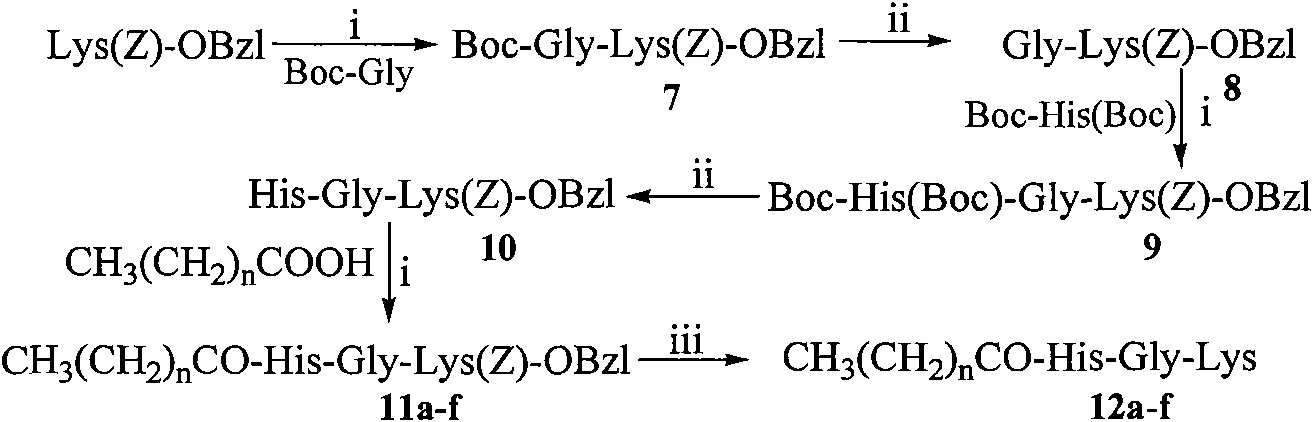Saturated fat chain acid His-Gly-AA tripeptide amide, and synthesis method and use thereof
A his-gly-aa, n-co-his-gly-aai technology, applied in the field of biomedicine, can solve the problems of cyclosporin A, such as poor water solubility, unsatisfactory dosage form or unsatisfactory curative effect, strong nephrotoxicity, etc., to achieve Excellent immunosuppressive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Preparation of Boc-Gly-Glu(OBzl)-OBzl
[0040]3.50 g (20.00 mmol) of Boc-Gly was dissolved in 200 ml of anhydrous THF, and 2.72 g (20.00 mmol) of N-hydroxybenzotriazole (HOBt) was added to the resulting solution under ice cooling to completely dissolve it. After 10 minutes 4.96 g (24.00 mmol) of dicyclohexylcarbodiimide (DCC) were added. Obtain reaction liquid (I), stand-by. Suspend 10.38g (20.00mmol) TosH·Glu(OBzl)-OBzl in 20ml anhydrous THF under ice-cooling, then add 1ml N-methylmorpholine (NMM) to adjust the pH to 8-9. Stir for 35 minutes to obtain the reaction solution (II), which is ready for use. The reaction solution (I) was added to the reaction solution (II) under ice bath, stirred for 1 h under ice bath, and then stirred at room temperature for 12 h. TLC (chloroform / methanol, 10:1) showed that Boc-Gly-OH disappeared. Dicyclohexylurea (DCU) was filtered off and THF was removed under reduced pressure. The residue was dissolved with 50 ml of ethyl ...
Embodiment 2
[0041] Example 2 Preparation of HCl Gly-Glu(OBzl)-OBzl
[0042] Dissolve 8.91g (18.38mmol) Boc-Gly-Glu(OBzl)-OBzl in 200ml 4mol / l hydrogen chloride-ethyl acetate solution, stir at room temperature for 2 hours, TLC (chloroform:methanol, 5:1) shows that the raw material point disappears , concentrated under reduced pressure to remove ethyl acetate, and the residue was repeatedly added with a small amount of ether for concentration under reduced pressure to remove hydrogen chloride gas. Finally, a small amount of diethyl ether was added to triturate the residue to obtain 6.94 g (90%) of the title compound as a colorless solid powder, which was directly used in the next reaction. ESI-MS(m / z): 385[M+H] + .
Embodiment 3
[0043] Example 3 Preparation of Boc-His(Boc)-Gly-Glu(OBzl)-OBzl
[0044] According to the method of Example 1, a beige oil was obtained from 5.42g (7.63mmol) Boc-His (Boc) and 6.94g (15.26mmol) HCl·Gly-Glu(OBzl)-OBzl. The obtained compound was purified by silica gel column chromatography to obtain 8.24 g of a colorless oily substance. Purification conditions: chloroform:methanol=100:1, the yield was 75%. ESI-MS(m / z): 722[M+H] + , [α] 20 D =-25.2 (c=1.0, CH 3 OH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com