Method for modeling production of fusion protein by utilizing trans-splicing of intein
A technology of fusion protein and fusion protein, which is applied in the field of model production of recombinant fusion protein, can solve the problems of environmental pollution, separation and purification difficulties, large length of fusion gene, etc., and achieve the effect of reducing production cost, simplifying operation process, and small gene length
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023] The method scheme of the present invention will be further specifically described by way of examples below.
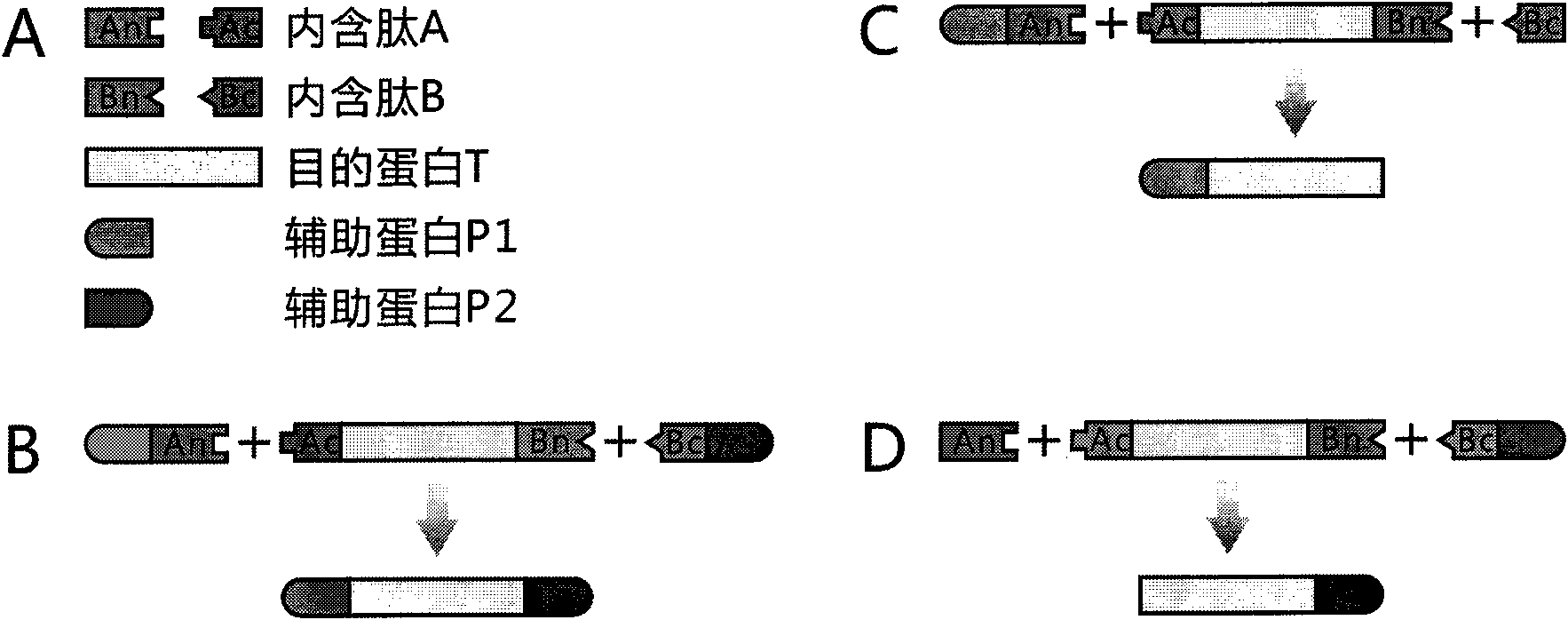
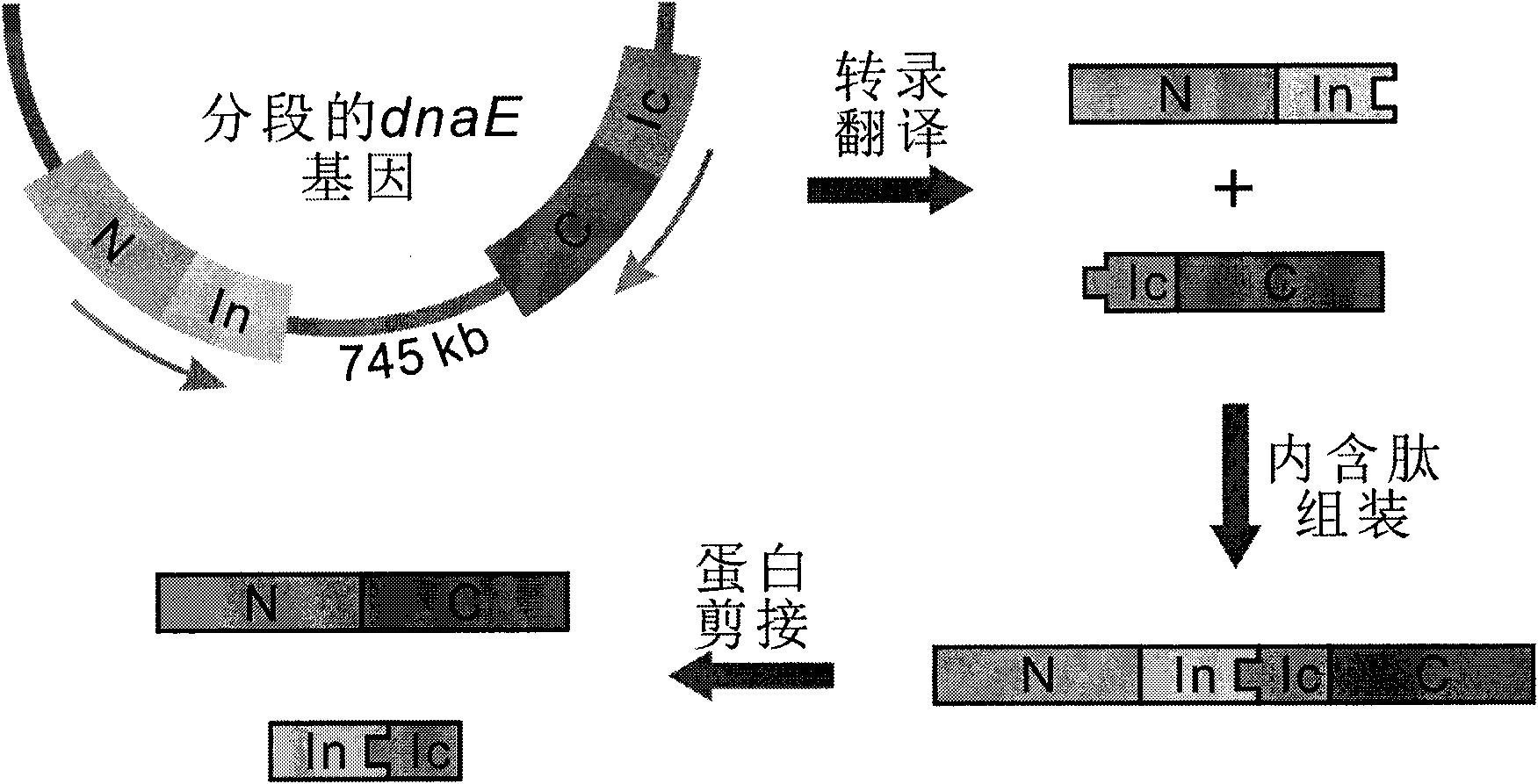
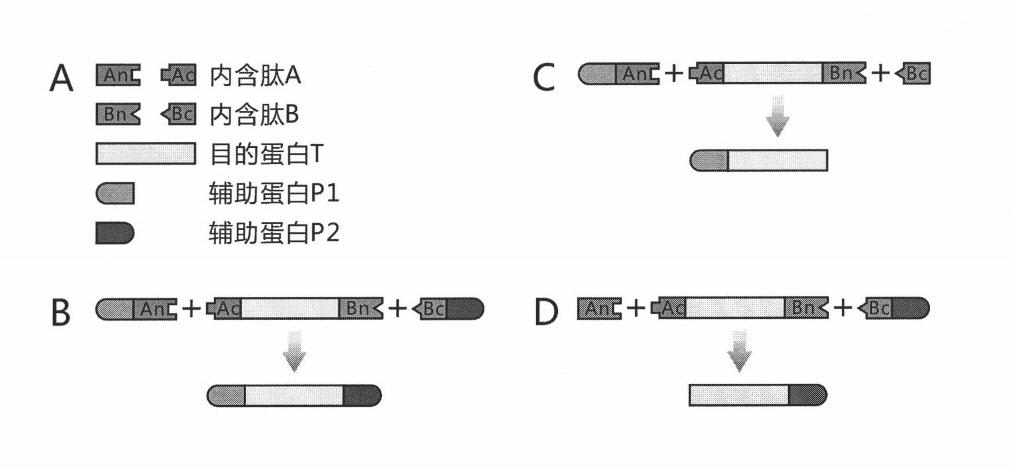
[0024] 1) Select the protein GUS encoded by the beta-D-glucuronidase gene of Escherichia coli str.K-12substr.MG1655 as the target protein T. See NCBI Reference Sequence NP_416134.1 for the specific sequence.
[0025] 2) Select the DnaE intein and DnaB intein of the cyanobacterium Synechocystis sp. PCC6803 (http: / / www.neb.com / neb / inteins.html).
[0026] The N-terminal sequence of the DnaE intein is as follows: CLSFGTEILT VEYGPLPIGK IVSEEINCSV YSVDPEGRVY TQAIAQWHDR GEQEVLEYEL EDGSVIRATS DHRFLTTDYQ LLAIEEIFAR QLDLLTLENI KQTEEALDNH RLPFPLLDAG TIK.
[0027] The carboxy-terminal sequence of the DnaE intein is as follows: MVKVIGRRSLGVQRIFDIGLPQDHNFLLANGAIAAN.
[0028]DnaB内含肽序列如下:CISGDSLISL ASTGKRVSIK DLLDEKDFEI WAINEQTMKL ESAKVSRVFC TGKKLVYILK TRLGRTIKAT ANHRFLTIDG WKRLDELSLK EHIALPRKLE SSSLQLMSDE ELGLLGHLIG DGCTLPRHAI QYTSNKIELA EKVVELAKAV FGDQINPRIS QERQWYQVYI PASY...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com