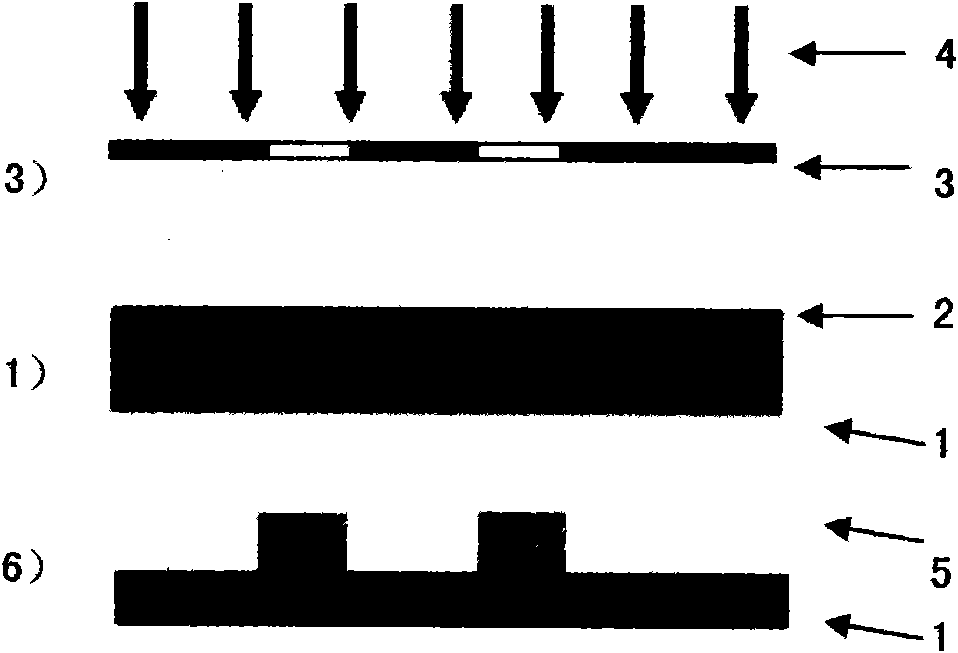
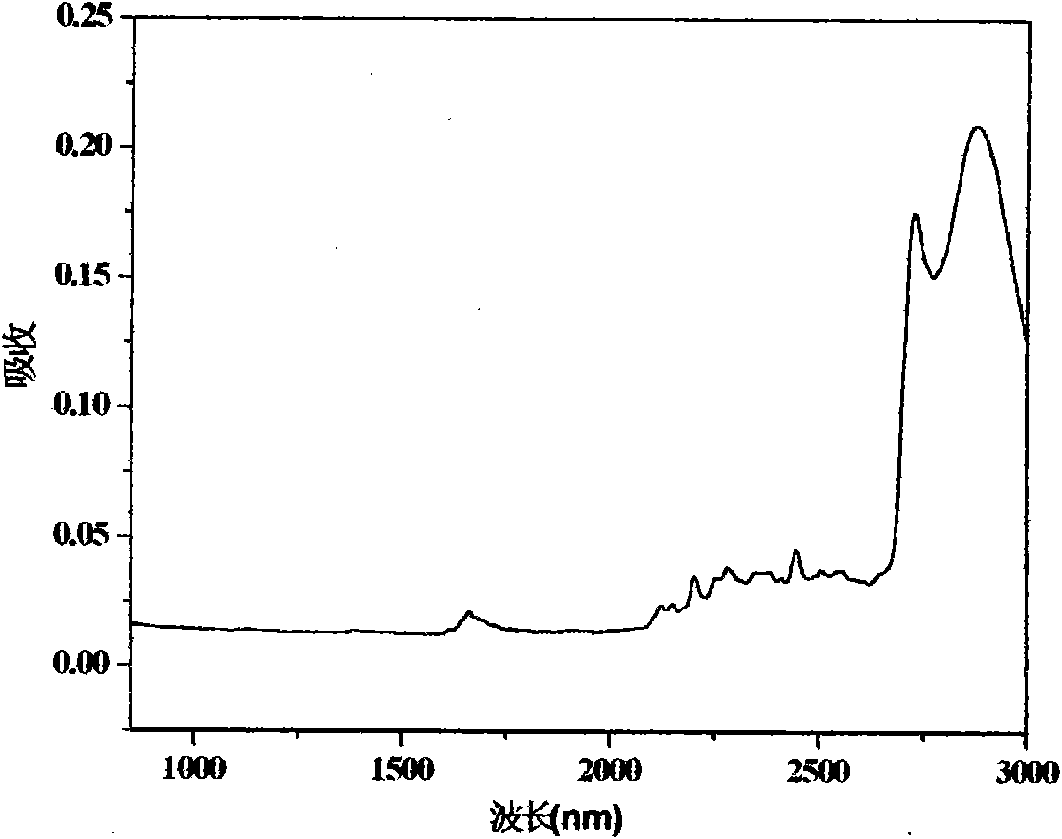
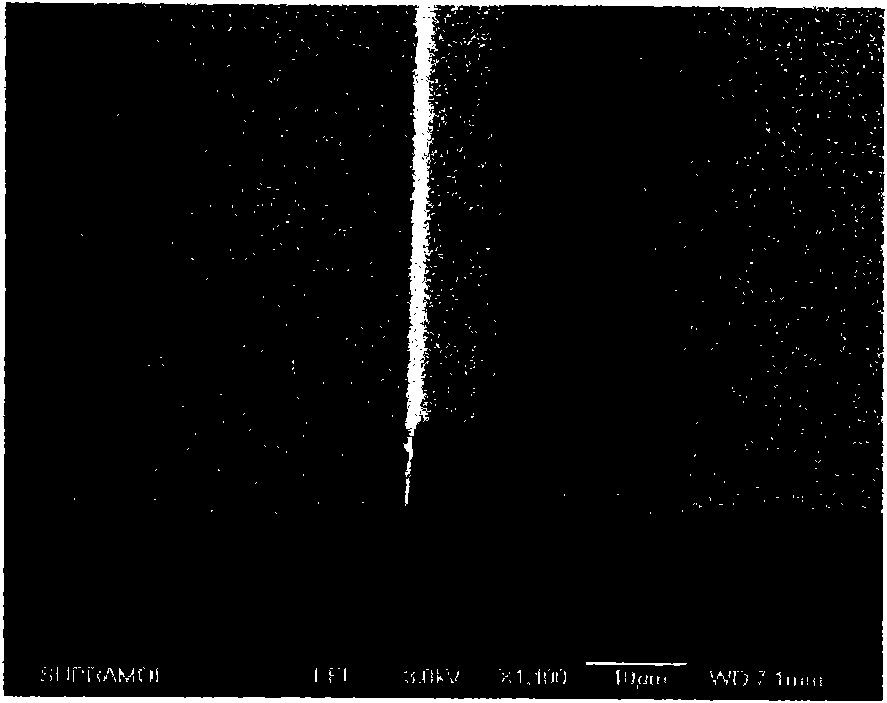
High-fluorine negative photoresist and application thereof to polymer optical waveguide device
A negative photoresist, polymer technology, applied in the direction of light guides, optical components, instruments, etc., can solve the problem of large light loss and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Dissolve 2.00g of pentafluorostyrene and 5.00g of fluorine-containing bisphenol A in 25ml of N,N-dimethylacetamide, and add the catalyst CaH 21.37g, CsF 0.13g, the mixture was heated up to 80°C, and reacted at this temperature for 18h. Filter to obtain a clear liquid. The solvent was evaporated. Column chromatography (ethyl acetate:petroleum ether=1:5) gave pure product. Dissolve the upper product in 25g of epichlorohydrin, catalyze it with solid caustic soda, add 0.04g of NaOH every 0.5h, and react for 8 hours in total. After the addition, keep at 60°C for 6h to complete the cyclization reaction. Steam unreacted epichlorohydrin, then extract with benzene, filter and evaporate benzene. The product from the previous step was dissolved in N,N-dimethylacetamide, 0.05 g of azobisisobutyronitrile was added, and the reaction was carried out at 60° C. for 6 h under nitrogen gas. The solvent is evaporated to obtain a high fluorine-containing epoxy resin with the following ...
Embodiment 2
[0033] Dissolve 2.00g of pentafluorostyrene and 3.40g of bisphenol A in 25ml of N,N-dimethylacetamide, add catalyst CaH 2 1.37g, CsF0.13g, the mixture was heated to 80°C and reacted at this temperature for 18h. Filter to obtain a clear liquid. The solvent was evaporated. Column chromatography (ethyl acetate:petroleum ether=1:4) gave pure product. Dissolve the upper product in 25g of epichlorohydrin, catalyze it with solid caustic soda, add 0.05g of NaOH every 0.5h, and react for a total of 8 hours. After the addition, keep at 60°C for 6h to complete the cyclization reaction. Steam unreacted epichlorohydrin, then extract with benzene, filter and evaporate benzene. The above product was dissolved in N,N-dimethylacetamide, 12 g of pentafluorostyrene was added, 0.10 g of initiator azobisisobutyronitrile was added, and the reaction was carried out at 60° C. for 6 h under nitrogen gas. The solvent is evaporated to obtain a high fluorine-containing epoxy resin with the followin...
Embodiment 3
[0037] Dissolve 2.00g of pentafluorostyrene and 5.00g of fluorine-containing bisphenol A in 25ml of N,N-dimethylacetamide, add the catalyst CaH 2 1.37g, CsF0.13g, the mixture was heated to 80°C and reacted at this temperature for 18h. Filter to obtain a clear liquid. The solvent was evaporated. Column chromatography (ethyl acetate:petroleum ether=1:5) gave pure product. Dissolve the upper product in 25g of epichlorohydrin, catalyze it with solid caustic soda, add 0.04g of NaOH every 0.5h, and react for 8 hours in total. After the addition, keep at 60°C for 6h to complete the cyclization reaction. Steam unreacted epichlorohydrin, then extract with benzene, filter and evaporate benzene. The above product was dissolved in N,N-dimethylacetamide, 15 g of pentafluorostyrene and 0.11 g of initiator azobisisobutyronitrile were added, and the reaction was carried out at 60° C. for 6 h under nitrogen gas. The solvent is evaporated to obtain a high fluorine-containing epoxy resin w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com