Morinidazole crystal and preparation method and medical application thereof
A morpholinidazole and crystal technology, applied in the field of morpholinidazole crystals and preparation thereof, can solve the problems of poor sensitivity of non-spore Gram-positive bacilli and the like, and achieve the effect of superior stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) 20g of morpholinidazole crude product is dissolved in 20ml of sulfuric acid aqueous solution of 3mo / L, stirs 2h and reacts and becomes salt, then adds gac 2g decolouring 0.5h, filters;
[0033] (2) adjust the pH to 7.5 with the filtrate obtained in the previous step, and the morpholinidazole is free to become a base and separate out, and filter;
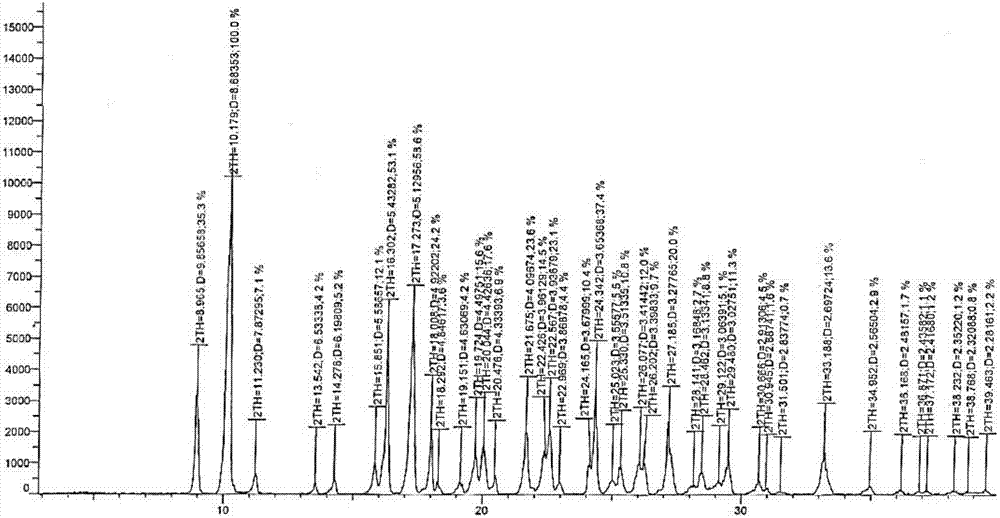
[0034] (3) Add the filter cake obtained in the previous step into 160ml of acetone, heat to 50°C, keep stirring for 0.5h, filter while it is hot, then cool to 30°C, stir and crystallize, filter, wash with ice water, and dry to obtain a yellow color Linidazole crystals 18g. After testing, the XRPD pattern of morpholinidazole crystal is as follows: figure 1 shown.
Embodiment 2
[0036] (1) 20g morpholinidazole crude product is dissolved in 20ml of aqueous nitric acid solution of 6mo / L, stirs 2h and reacts into salt, then adds gac 2g decolouring 0.5h, filters;
[0037] (2) adjust the pH to 8.0 with the filtrate obtained in the previous step, and the morpholinidazole is free to become a base and separate out, and filter;
[0038] (3) Add the filter cake obtained in the previous step into 200ml of acetone, heat to 55°C, keep stirring for 0.5h, filter while it is hot, then cool to 10°C, stir and crystallize, filter, wash with ice water, and dry to get yellow Linidazole crystals 18g. After testing, the XRPD pattern of morpholinidazole crystals is basically as follows: figure 1 shown.
Embodiment 3
[0040] (1) 20g of morpholinidazole crude product is dissolved in 20ml of aqueous hydrochloric acid solution of 6mo / L, stirs 2h and reacts into salt, then adds gac 2g decolouring 0.5h, filters;
[0041] (2) adjust the pH to 8.0 with the filtrate obtained in the previous step, and the morpholinidazole is free to become a base and separate out, and filter;
[0042] (3) Add the filter cake obtained in the previous step into 200ml of acetone, heat to 55°C, keep stirring for 0.5h, filter while hot, then cool to 20°C, stir to crystallize, filter, wash with ice water, and dry to get yellow Linidazole crystals 19g. After testing, the XRPD pattern of morpholinidazole crystals is basically as follows: figure 1 shown.
[0043] 2. Stability research
[0044] The crystal form samples obtained in Example 1 were placed under high temperature (60°C), high humidity (25°C / RH92.5%), and light conditions for 30 days, and the crystal form detection results are shown in the table below:
[0045]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com