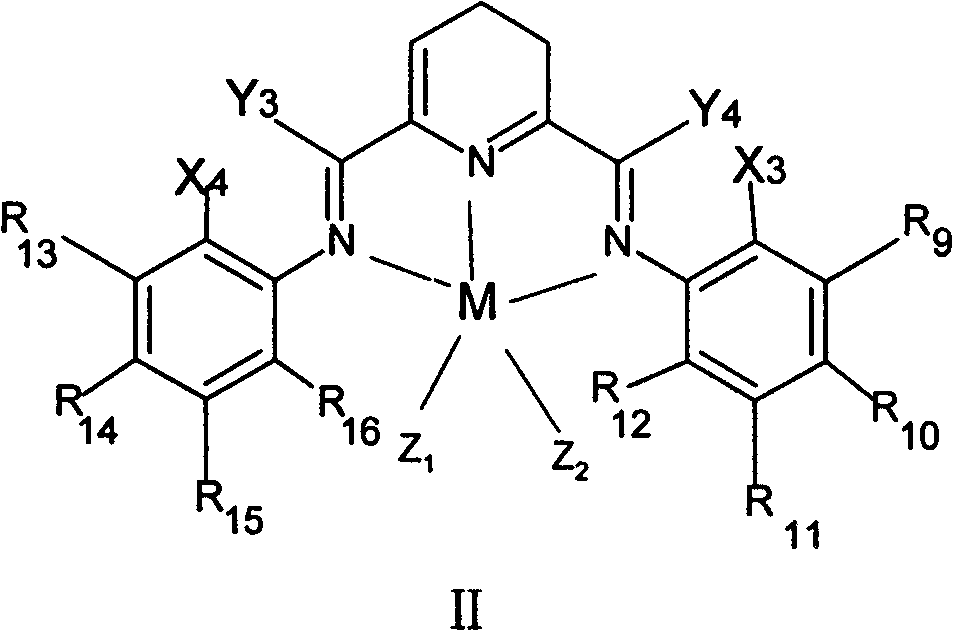
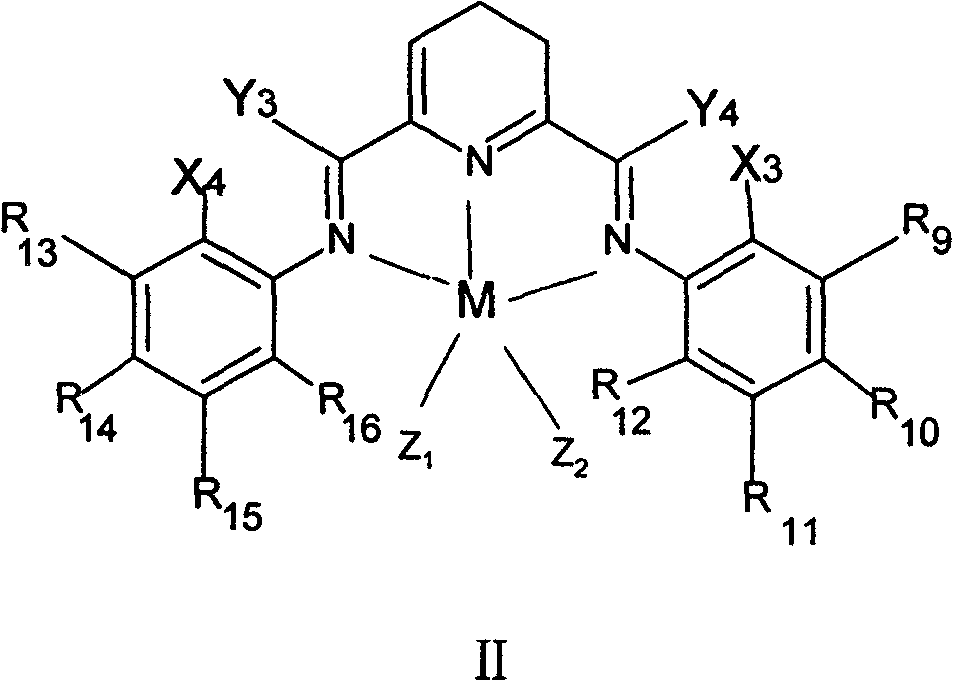
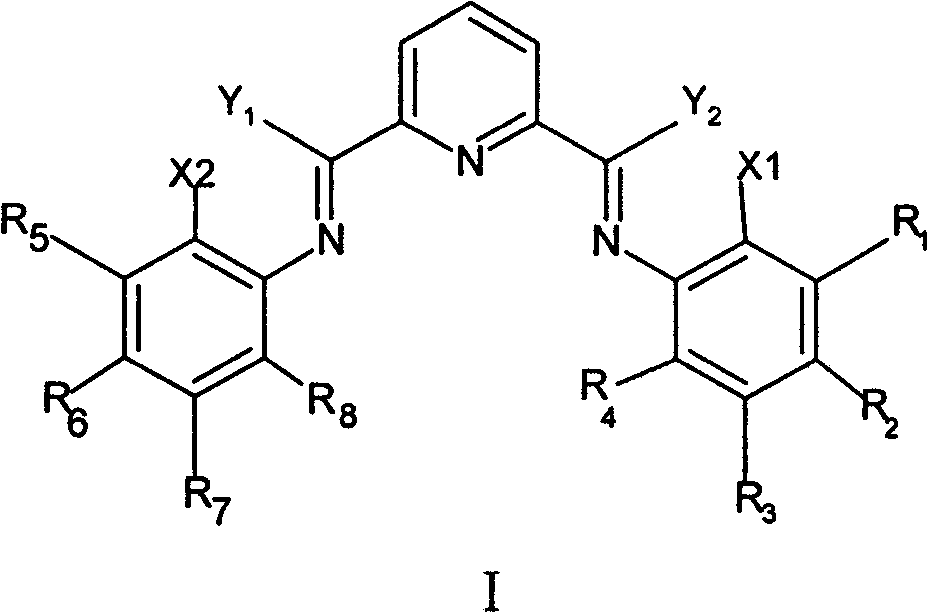
Method for preparing linear alpha-olefin
An olefin and linear technology, which is applied in the field of preparing linear α-olefins, can solve the problems of narrow distribution range of α-olefin products, increased investment cost and operating cost of production equipment, and inability to flexibly adjust content, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] In a 50ml flask, add 4.5g 2-methyl-4-methoxyaniline, 1.63g 2,6-diacetylpyridine, 20ml toluene, 3g molecular sieve and 0.5g silica-alumina catalyst, after reacting for 18 hours, filter , and washed with 20ml of toluene, the solvent was removed under reduced pressure, and the product was recrystallized in methanol to obtain light yellow ligand L1{2,6-bis-[(2-methyl-4-methoxyanilinoethyl)pyridine] }, yield 80%.
[0052] 1 H-NMR (CDCl 3 / TMS, δ): 8.26 (d, 2H), 8.02 (t, 1H), 6.99 (d, 4H), 6.72 (m, 2H), 3.79 (s, 6H), 2.34 (s, 6H). Elemental analysis: calculated value (mass percentage, %) C: 74.80, H: 6.73, N: 10.47, O: 7.98; measured value (mass percentage, %) C: 74.23, H: 7.03, N: 10.08, O: 8.64 .
Embodiment 2
[0054] In a 50ml flask, add 4.2g 2-chloro-4-methylaniline, 1.63g 2,6-diacetylpyridine, 20ml toluene, 3g molecular sieve and 0.5g silica-alumina catalyst, after reacting for 24 hours, filter, and use 20ml of toluene was washed, the solvent was removed under reduced pressure, and the product was recrystallized in methanol to obtain light yellow ligand L 2 {2,6-Di-[(2-chloro-4-methylanilinoethyl)pyridine]}, yield 82%.
[0055] 1 H-NMR (CDCl 3 / TMS, δ): 8.23 (d, 2H), 7.99 (t, 1H), 7.07 (s, 2H), 6.88 (d, 2H), 6.69 (d, 2H), 2.80 (s, 6H), 2.22 ( s, 6H). Elemental analysis: calculated value (mass percentage, %) C: 67.33, H: 5.16, N: 10.24, Cl: 17.27; measured value (mass percentage, %) C: 66.81, H: 5.36, N: 9.77, Cl: 18.06 .
Embodiment 3
[0057] In a 50ml flask, add 1.5g 2,6-diacetylpyridine, 5.2g 2,6 difluoroaniline, 20ml toluene, 3g molecular sieve and 0.5g silica aluminum catalyst, after reacting for 12 hours, filter, and wash with 20ml toluene, The solvent was removed under reduced pressure, and the product was recrystallized in methanol to give light yellow ligand L 3 {2,6-Di-[(2,6-difluoroanilinoethyl)pyridine]}, yield 78%.
[0058] 1 H-NMR (CDCl 3 ): δ=8.47 (d, 2H), 7.93 (t, 1H), 7.07 (t, 4H), 6.99 (d, 2H), 2.46 (s, 6H,). Elemental analysis: calculated value (mass percentage, % ): C, 65.45; H, 3.92; N, 10.90; Measured value (mass percentage, %): C, 65.61; H, 4.02; N, 10.78.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com