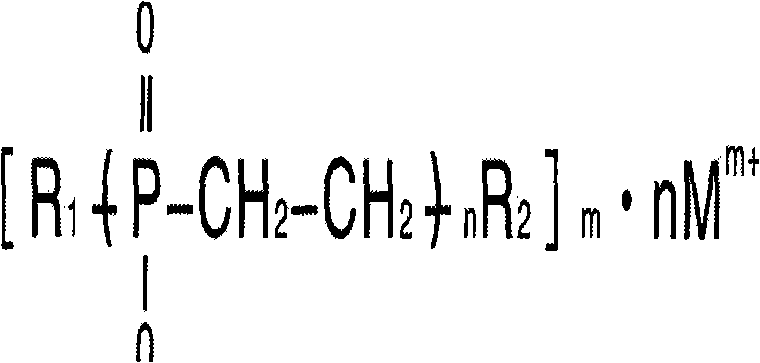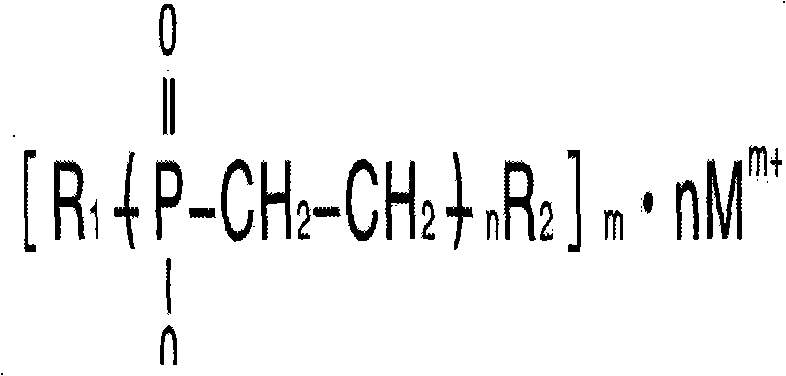Alkyl phosphinate polymer and preparation method and application thereof
A technology of alkyl phosphinate and alkyl phosphinate, which is applied in the field of alkyl phosphinate polymer and its preparation and application, and can solve the problems of insufficient thermal stability of flame retardants and difficulties in meeting processing requirements, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] An aluminum dialkylphosphinate polymer is first prepared. For this, a mixture of 150 KG of sodium hypophosphite and 750 kg of deionized water was charged into a 2000 L jacketed pressure reactor. Once the reaction mixture had been heated to 100°C, acetylene was added through a pressure relief valve set at 3 bar until saturation was reached in the reactor. 2.0KG2, 2' azobis (N, N, a dimethylene isobutyromide) dihydrochloride solution in 200KG water is uniformly fed to the in the mixture. A total of 18.5KG of acetylene was consumed. Acetylene was replaced by ethylene, which was added through a pressure relief valve set at 6 bar until saturation was reached in the reactor. The catalyst is uniformly fed into the mixture at a temperature of 100---105°C. Total reaction 10h. Consumption of ethylene 39.6KG. The reactor was depressurized, and after cooling to 80 °C. Within 2 hours, add 290KG of 50% Al-containing 2 o 3 16.53wt% Al 2 (SO 4 ) 3 aqueous solution. The res...
Embodiment 2
[0042] An aluminum dialkylphosphinate polymer is first prepared. For this, a mixture of 150 KG of sodium hypophosphite and 750 kg of deionized water was charged into a 2000 L jacketed pressure reactor. Once the reaction mixture had been heated to 100°C, acetylene was added through a pressure relief valve set at 3 bar until saturation was reached in the reactor. 2.0KG2, 2' azobis (N, N, a dimethylene isobutyromide) dihydrochloride solution in 200KG water is uniformly fed to the in the mixture. A total of 24.5KG of acetylene was consumed. Acetylene was replaced by ethylene, which was added through a pressure relief valve set at 6 bar until saturation was reached in the reactor. The catalyst is uniformly fed into the mixture at a temperature of 100---105°C. Total reaction 10h. Consumption of ethylene 26.5KG. The reactor was depressurized, and after cooling to 80 °C. Within 2 hours, add 290KG of 50% Al-containing 2 o 3 16.53wt% Al 2 (SO 4 ) 3 aqueous solution. The res...
Embodiment 3
[0044] An aluminum dialkylphosphinate polymer is first prepared. For this, a mixture of 150 KG of sodium hypophosphite and 750 kg of deionized water was charged into a 2000 L jacketed pressure reactor. Once the reaction mixture had been heated to 100°C, acetylene was added through a pressure relief valve set at 3 bar until saturation was reached in the reactor. A solution of 2.0KG potassium persulfate in 200KG water was uniformly fed into the mixture under continuous stirring at a temperature of 100---105°C. A total of 27.6KG of acetylene was consumed. Acetylene was replaced by ethylene, which was added through a pressure relief valve set at 6 bar until saturation was reached in the reactor. The catalyst is uniformly fed into the mixture at a temperature of 100---105°C. Total reaction 10h. Consumption of ethylene 20.0KG. The reactor was depressurized, and after cooling to 80 °C. Within 2 hours, add 290KG of 50% Al-containing 2 o 3 16.53wt% Al 2 (SO 4 ) 2 aqueous sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com