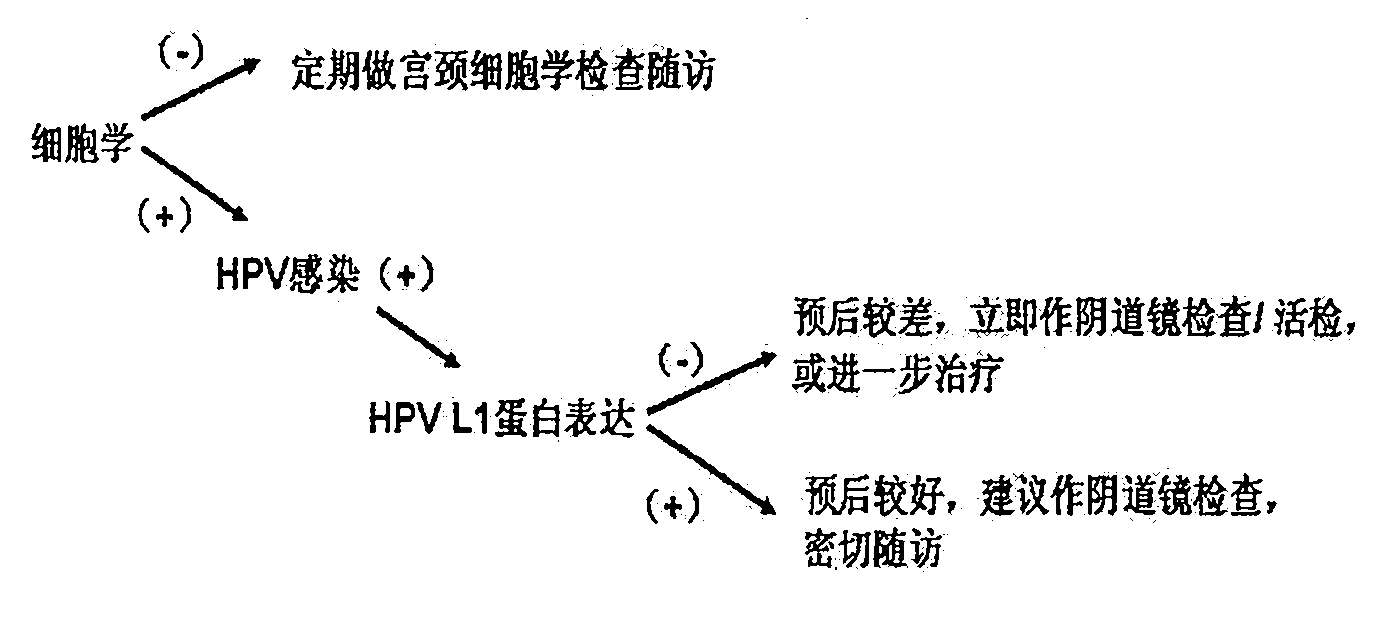
Human papilloma virus (HPV) capsid protein L1 polypeptide and preparation and application thereof
A technology of capsid protein and papilloma, applied in the field of peptides, can solve the problems of aggravating the psychological and economic burden of patients and overtreatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1. Screening and peptide synthesis of conserved epitopes of 18 low-risk and high-risk HPV subtypes:
[0036] Through computer simulation, L1 protein epitope prediction and multiple sequences of 18 HPV subtypes (6, 11, 16, 18, 52, 58, etc.) Alignment, and using the naccess program to calculate the liquid-phase accessible area of the corresponding amino acid residues, we screened a very conservative peptide located on the surface of the protein (N---Cys Thr Leu Thr Ala Asp Val Met Thr Tyr Ile His--- C), and entrusted Beijing Saibaisheng Biotechnology Co., Ltd. to synthesize the peptide and its cross-linked product (Cys Thr Leu Thr Ala Asp Val Met Thr Tyr Ile His--KLH), with a purity of >90%.
[0037] 2. Preparation of antiserum:
[0038] In the primary immunization experiment group, Freund's complete adjuvant was mixed with the synthetic immunogen Cys Thr Leu Thr Ala Asp Val Met Thr Tyr Ile His-KLH protein in equal amounts, and stirred on a magnetic stirrer for 30 minu...
Embodiment 2
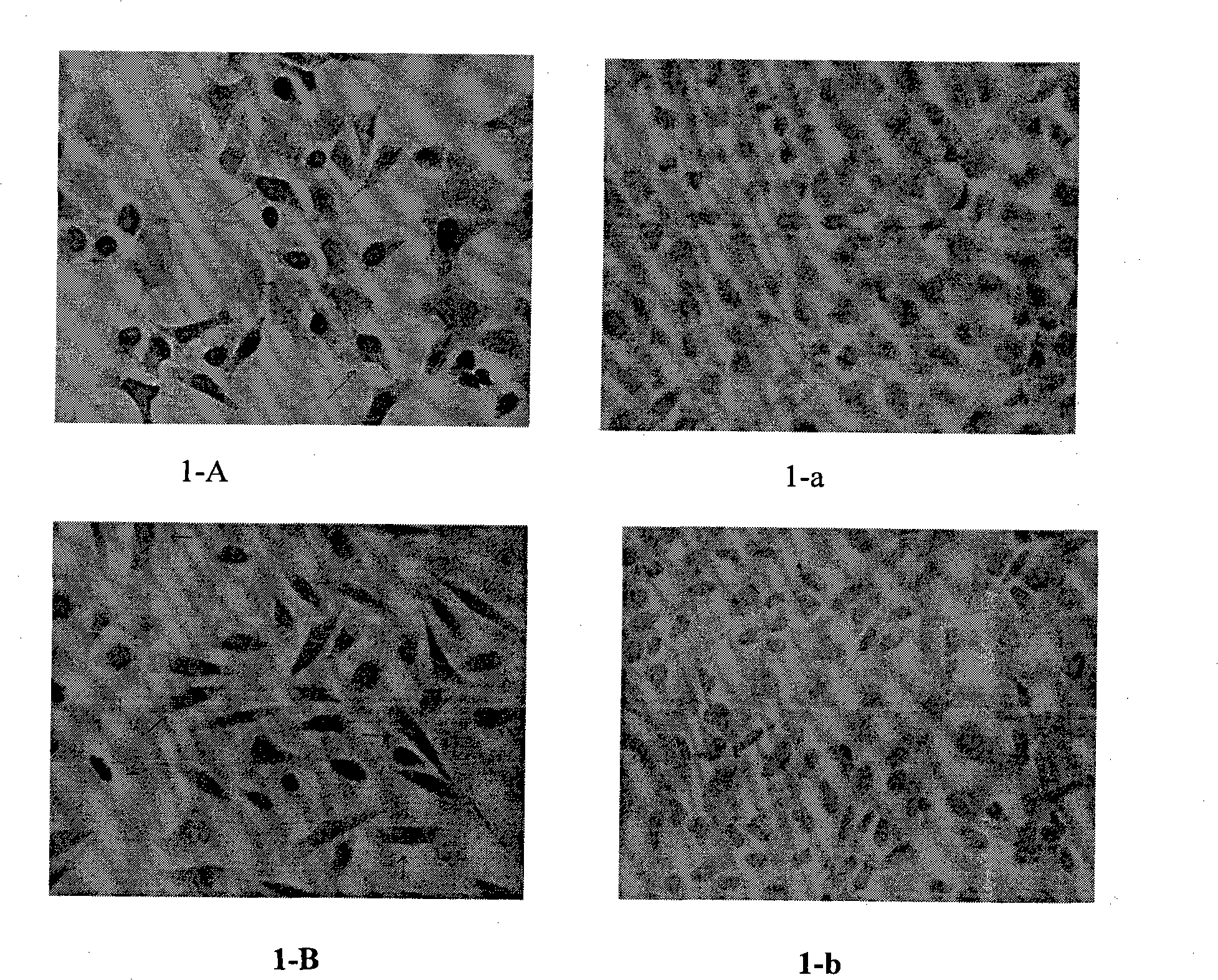
[0040] Immunocytochemical detection of the reactivity of anti-polypeptide antiserum to cells infected with HPV virus: HeLa (cervical adenocarcinoma cell line infected with HPV18), Siha (cervical squamous cell line infected with HPV16) in logarithmic growth phase After cells were digested with trypsin, the cell suspension was seeded on coverslips placed in 24-well plates, cultured overnight at 37°C in a CO2 incubator, and fixed with 4% paraformaldehyde for 15 minutes when the cells covered about 70% of the slides. Wash with PBS for 5min×3 times, 3%H 2 o 2 Eliminate endogenous peroxidase for 30 min, PBS 5 min x 3 times, punch with 0.1% Triton for 15 min, PBS 5 min x 3 times, block with goat serum blocking solution for 30 min, add 1:500 diluted rabbit serum at 4°C overnight, wash with PBS 5min×3 times, then drop goat anti-rabbit secondary antibody working solution for 15min, wash with PBS for 5min×3 times, add horseradish-labeled streptavidin working solution, wash with PBS for ...
Embodiment 3
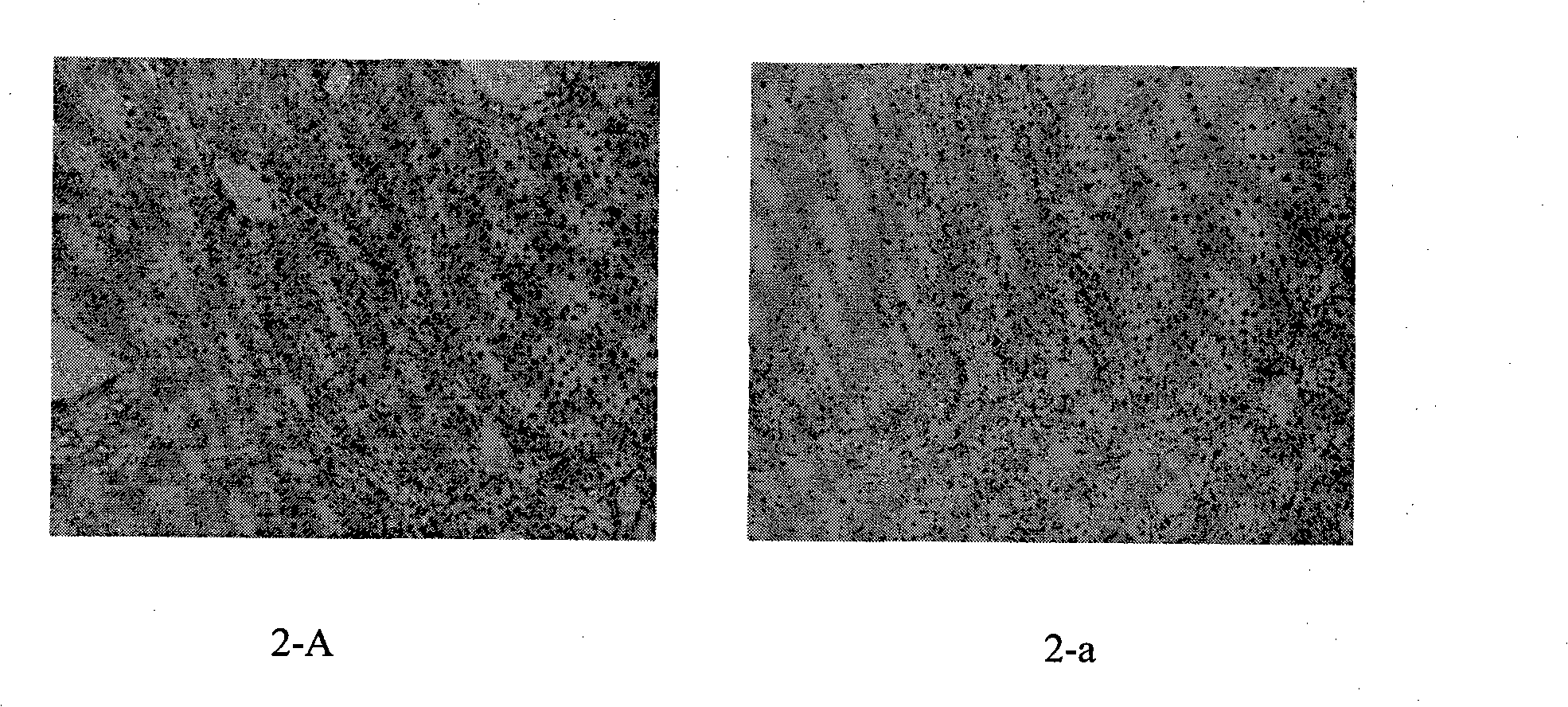
[0043]Immunohistochemical detection of the reactivity of anti-polypeptide antiserum to HPV-positive clinical specimens: tissue specimens identified as condyloma acuminata, CIN, cervicitis and HPV-infected positive by the clinical pathology department were fixed in 10% formaldehyde, embedded in paraffin, sliced, After deparaffinization and hydration, repair with 0.01mol / lpH6.0 citrate buffer at 100°C for 20min, cool to room temperature naturally, wash with PBS for 5min×3 times, 3%H 2 o 2 Eliminate endogenous peroxidase for 30 min, PBS 5 min x 3 times, block with goat serum blocking solution for 30 min, add 1:500 diluted rabbit serum overnight at 4°C, wash with PBS for 5 min x 3 times, then add goat anti-rabbit II dropwise Anti-working solution for 15 minutes, wash with PBS for 5 minutes×3 times, add horseradish-labeled streptavidin working solution, wash with PBS for 5 minutes×3 times, develop color with DAB for 3 minutes, stop the reaction with tap water, counterstain with hem...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com