Small molecular compound N-alkyl acyl cystamine for simulating function of folding enzyme, a preparation method thereof and method for assisting protein oxidizing and refolding
A technology of small molecule compound, alkyl cysteine, which is applied in the field of protein renaturation in biotechnology, can solve the problem of not being able to effectively simulate specific interactions, not having the specificity of protein oxidation, and the catalytic effect of small molecule mimics It can save the time and cost of renaturation, reduce the cost of renaturation, and assist the oxidative renaturation of proteins.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
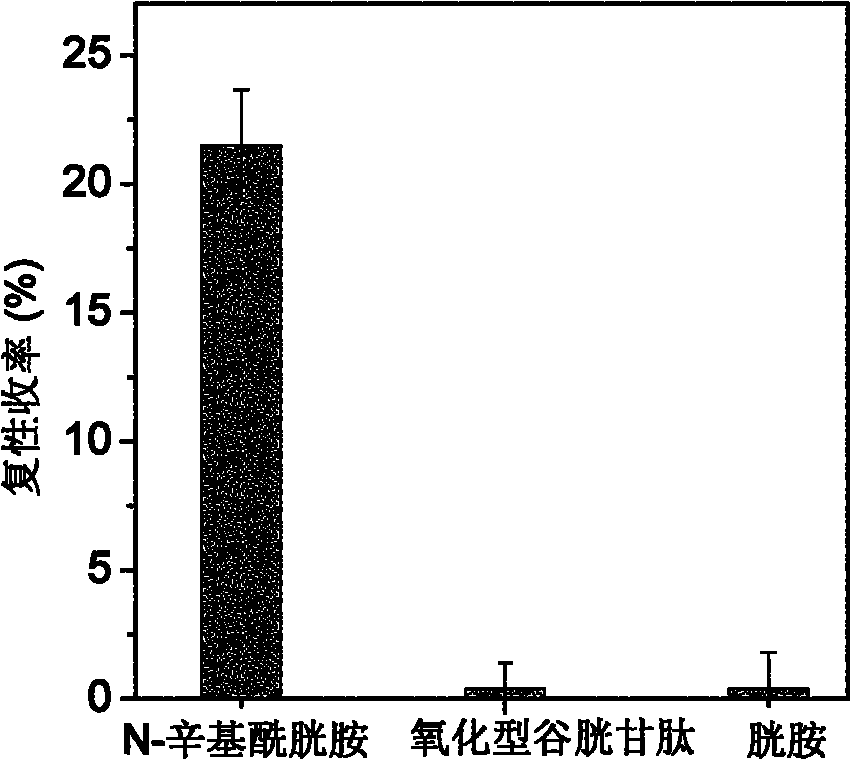
[0030] Example 1: Synthesis of N-octylcystamine and its application in renaturation of assisted reduction denatured lysozyme
[0031]Weigh 0.225g of cystamine dihydrochloride and dissolve it in 10mL of deionized water to obtain a 0.1mol / L cystamine solution, then drop in 3.18μL of n-octanoic acid to make the concentration 0.002mol / L, mix well, and add 2mol / L NaOH The pH of the solution was adjusted to 6.3, and then 0.384 g of 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride was added thereto to make the final concentration 0.2 mol / L, and mixed quickly and uniformly. The reaction system was placed in a constant temperature water bath shaker at 25°C, and reacted for 4 hours to obtain a crude product. The crude product was filtered through a 0.45 μm water-based microporous membrane, and then separated by high-performance liquid chromatography on a C18 reverse-phase column. The mobile phase A was an aqueous phase containing 0.1% trifluoroacetic acid (TFA), and B was acet...
Embodiment 2
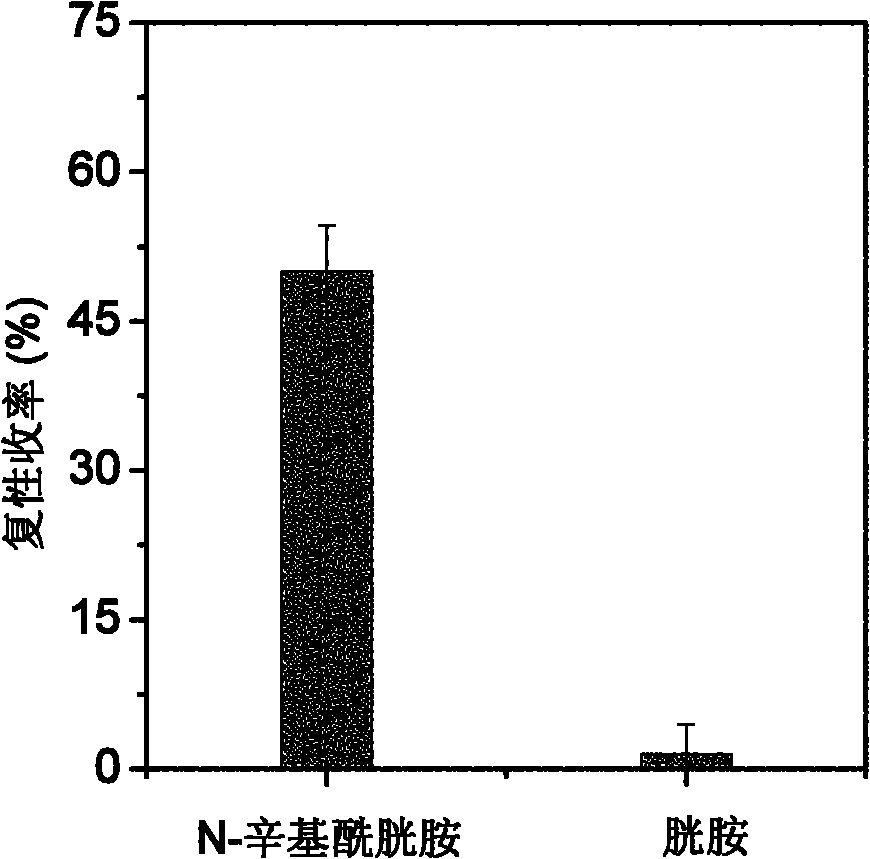
[0033] Embodiment 2: the renaturation of N-octylcystamine assisted reduction denatured lysozyme synthesized in embodiment 1
[0034] The N-octylcystamine prepared in Example 1 was used to assist the renaturation of the reduced denatured lysozyme. 14.3mg of lysozyme was dissolved in 1.0mL containing 8mol / L urea, 100mmol / L DTT, 100mmol / L tris-hydrochloride (Tris-HCl) and 1mmol / L EDTA, in the denaturing buffer solution of pH 8.5, Mix well and reduce and denature at 40°C for 3 hours; after the denaturation is completed, the residual DTT content is 84mmol / L as determined by C18 reverse phase chromatography; the obtained denatured lysozyme is diluted 5 times with DTT-free denaturing buffer Denatured protein samples. The refolding buffer was composed of 100mmol / L Tris-HCl, 2mol / L urea, 1mmol / L EDTA and 0.2mmol / L N-octylcystamine or cystamine, and the pH of the solution was 8.5. The denatured protein sample was diluted 40 times with refolding solution, the concentration of lysozyme ...
Embodiment 3
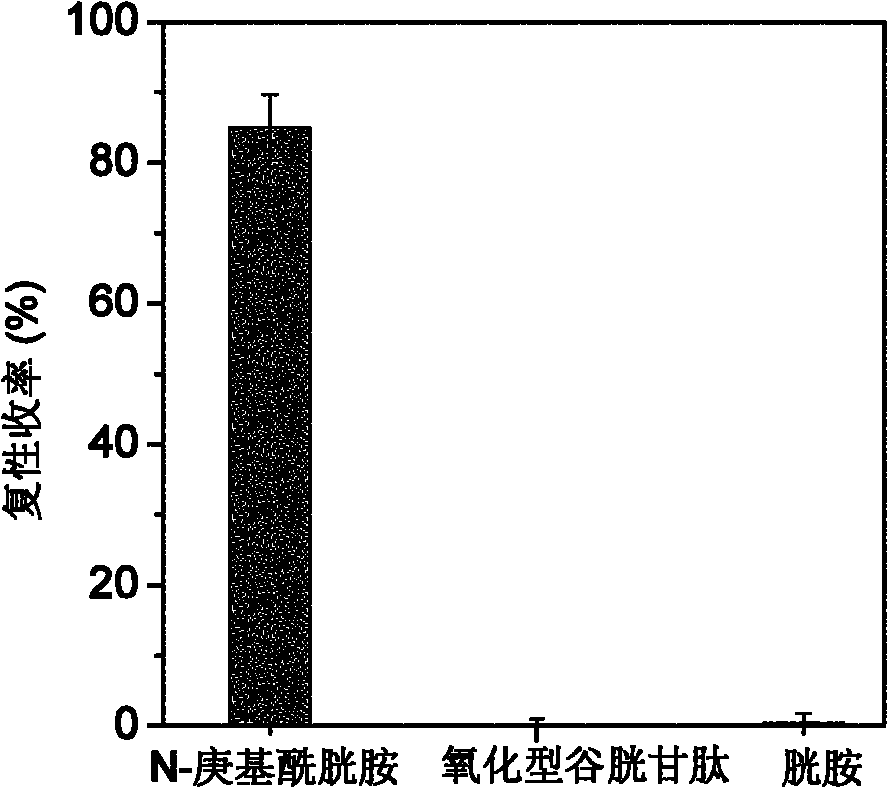
[0035] Example 3: Synthesis of N-heptylcystamine and its renaturation for auxiliary reduction of denatured ribonuclease A
[0036] Weigh 2.25g of cystamine dihydrochloride and dissolve it with 10mL of deionized water to obtain a 1mol / L cystamine solution, then add 28.6μL of n-heptanoic acid dropwise to make the concentration 0.02mol / L, mix well, and dissolve with 5mol / L NaOH The pH of the solution was adjusted to 6.5, and then 0.192 g of 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride was added thereto to make the final concentration 0.1 mol / L, and mixed quickly and uniformly. The reaction system was placed in a constant temperature water bath shaker at 15°C, and reacted for 6 hours to obtain a crude product. The crude product was filtered through a 0.45 μm water-based microporous membrane, and then separated by high-performance liquid chromatography on a C18 reverse-phase column. The mobile phase A was an aqueous phase containing 0.1% trifluoroacetic acid (TFA), an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com