Preparation method for recombinant human granulocyte colony stimulating factor
A technology of colony-stimulating factor and granulocytes, applied in the direction of colony-stimulating factor, cytokine/lymphokine/interferon, animal/human protein, etc., can solve the problems of cumbersome operation, low yield and high equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
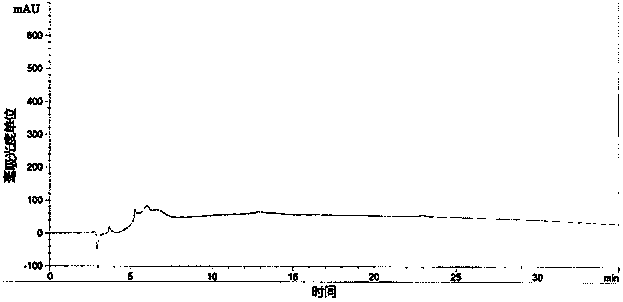
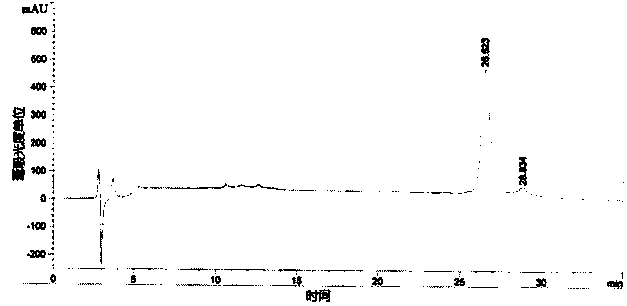
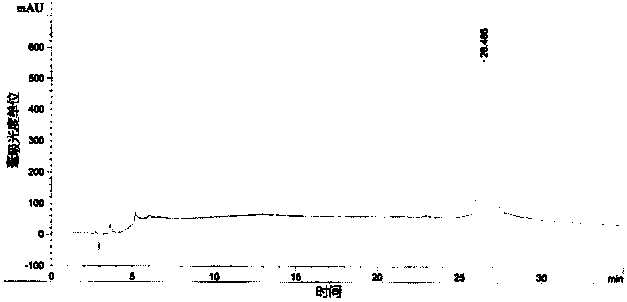
[0032] Acquisition of rhG-CSF inclusion bodies
[0033] The rhG-CSF genetically engineered bacteria (engineering strain pBVQG8 / MM294, provided by the Chinese Academy of Military Medical Sciences) was fermented in a conventional high-density manner (the engineering bacteria were fermented in a fed-batch culture method, and when the cell density reached OD value 30, The engineered bacteria were induced to ferment to express exogenous proteins; among them, the feed medium formula was: yeast extract powder 450g / L, glucose 500g / L; the fermentation medium formula was: peptone 12g / L, yeast extract 24g / L, Glycerol 4ml / L, Glucose 20g / L, Potassium dihydrogen phosphate 2.31g / L, Dipotassium hydrogen phosphate 12.54g / L, Magnesium sulfate 0.75g / L), the bacteria were collected by 8000g centrifugal force.
[0034] The collected wet bacteria were placed at -20°C and freeze-thawed once. Take 400 grams of freeze-thawed bacteria and suspend them in the lysate (20mM Tris-HCl, EDTA1mM, DTT1.5g / L, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com