Small molecular compound N-alkyl acyl cystamine for simulating function of folding enzyme, a preparation method thereof and method for assisting protein oxidizing and refolding
A technology of small molecular compound, alkyl cystamine, applied in the field of protein renaturation in biotechnology, to achieve the effect of improving speed, high renaturation yield and reducing renaturation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
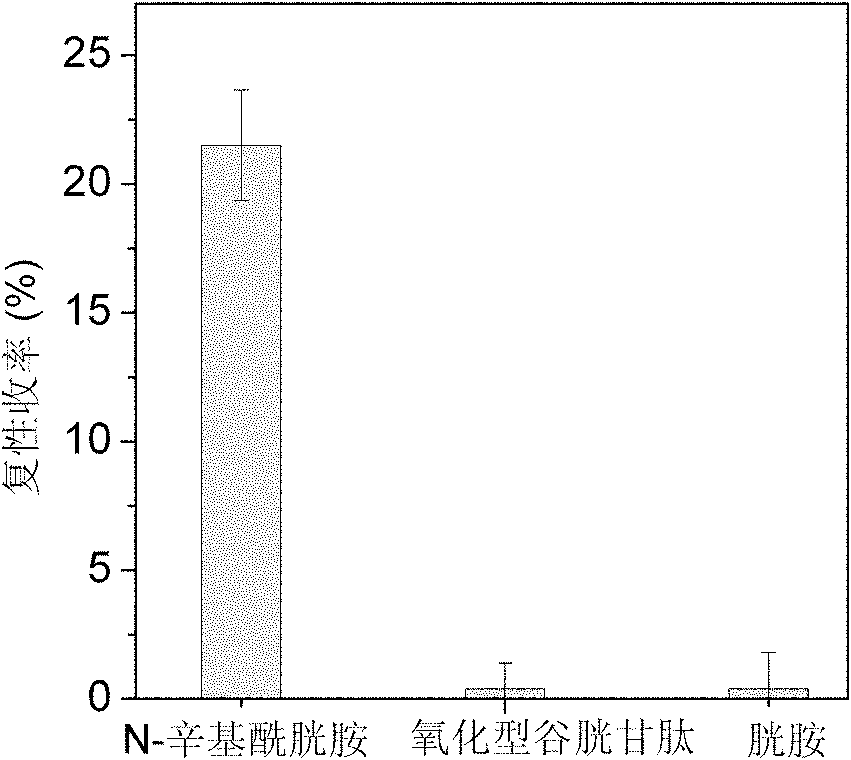
[0030] Example 1: Synthesis of N-octylcystamine and its application in renaturation of assisted reduction denatured lysozyme
[0031]Weigh 0.225g of cystamine dihydrochloride and dissolve it in 10mL of deionized water to obtain a 0.1mol / L cystamine solution, then drop in 3.18μL of n-octanoic acid to make the concentration 0.002mol / L, mix well, and add 2mol / L NaOH The pH of the solution was adjusted to 6.3, and then 0.384 g of 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride was added thereto to make the final concentration 0.2 mol / L, and mixed quickly and uniformly. The reaction system was placed in a constant temperature water bath shaker at 25°C, and reacted for 4 hours to obtain a crude product. The crude product was filtered through a 0.45 μm water-based microporous membrane, and then separated by high-performance liquid chromatography on a C18 reverse-phase column. The mobile phase A was an aqueous phase containing 0.1% trifluoroacetic acid (TFA), and B was acet...
Embodiment 2
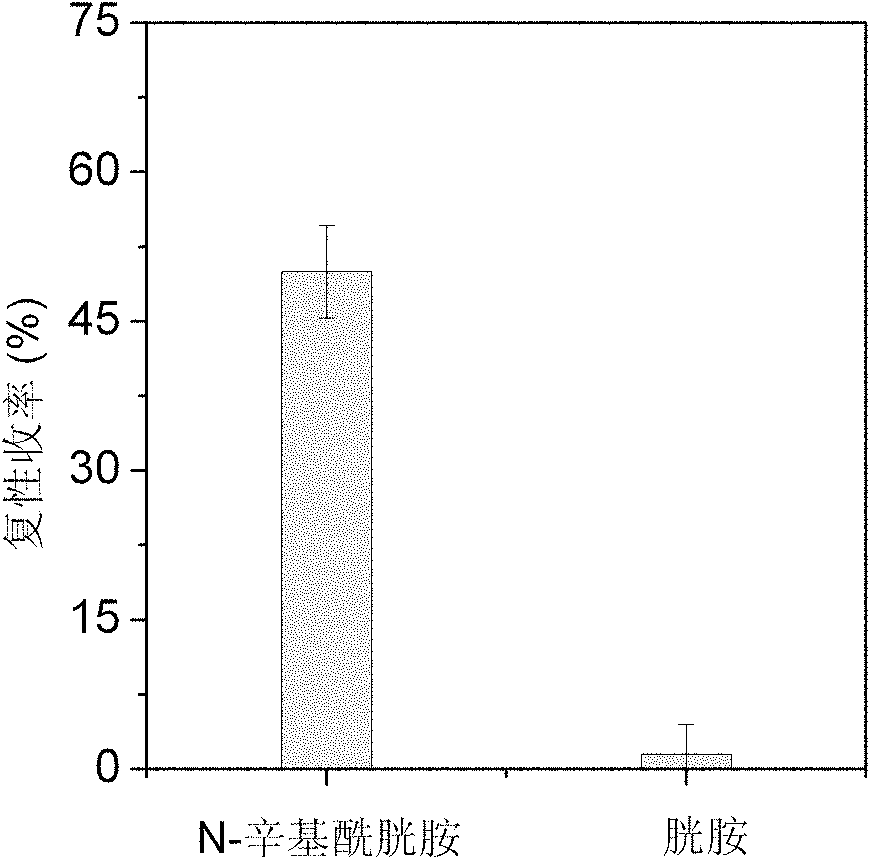
[0033] Embodiment 2: the renaturation of N-octylcystamine assisted reduction denatured lysozyme synthesized in embodiment 1
[0034] The N-octylcystamine prepared in Example 1 was used to assist the renaturation of the reduced denatured lysozyme. 14.3mg of lysozyme was dissolved in 1.0mL containing 8mol / L urea, 100mmol / L DTT, 100mmol / L tris-hydrochloride (Tris-HCl) and 1mmol / L EDTA, in the denaturing buffer solution of pH 8.5, Mix well and reduce and denature at 40°C for 3 hours; after the denaturation is completed, the residual DTT content is 84mmol / L as determined by C18 reverse phase chromatography; the obtained denatured lysozyme is diluted 5 times with DTT-free denaturing buffer Denatured protein samples. The refolding buffer was composed of 100mmol / L Tris-HCl, 2mol / L urea, 1mmol / L EDTA and 0.2mmol / L N-octylcystamine or cystamine, and the pH of the solution was 8.5. The denatured protein sample was diluted 40 times with refolding solution, the concentration of lysozyme ...
Embodiment 3
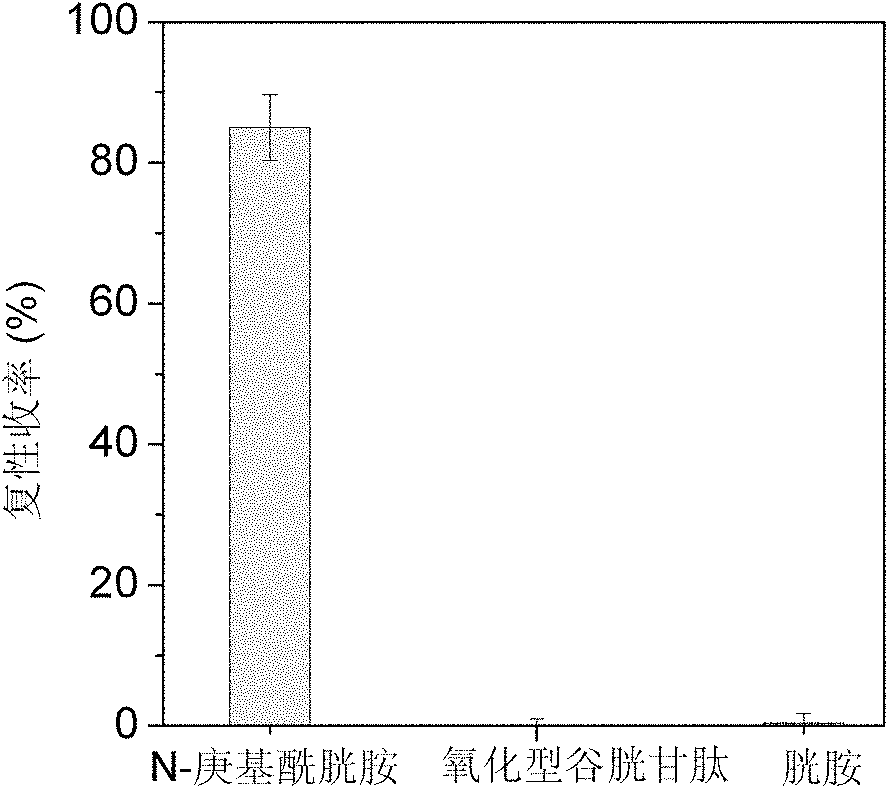
[0035] Example 3: Synthesis of N-heptylcystamine and its renaturation for auxiliary reduction of denatured ribonuclease A
[0036] Weigh 2.25g of cystamine dihydrochloride and dissolve it with 10mL of deionized water to obtain a 1mol / L cystamine solution, then add 28.6μL of n-heptanoic acid dropwise to make the concentration 0.02mol / L, mix well, and dissolve with 5mol / L NaOH The pH of the solution was adjusted to 6.5, and then 0.192 g of 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride was added thereto to make the final concentration 0.1 mol / L, and mixed quickly and uniformly. The reaction system was placed in a constant temperature water bath shaker at 15°C, and reacted for 6 hours to obtain a crude product. The crude product was filtered through a 0.45 μm water-based microporous membrane, and then separated by high-performance liquid chromatography on a C18 reverse-phase column. The mobile phase A was an aqueous phase containing 0.1% trifluoroacetic acid (TFA), an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com