Preparation method of recombinant tumor specificity antiapoptotic factors with activity and application of products thereof
A tumor-specific, apoptotic factor technology, which is applied in the field of biopharmaceutical preparation, can solve problems such as incapable of industrialization, high toxicity and side effects of drugs, and impossibility of large-scale application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
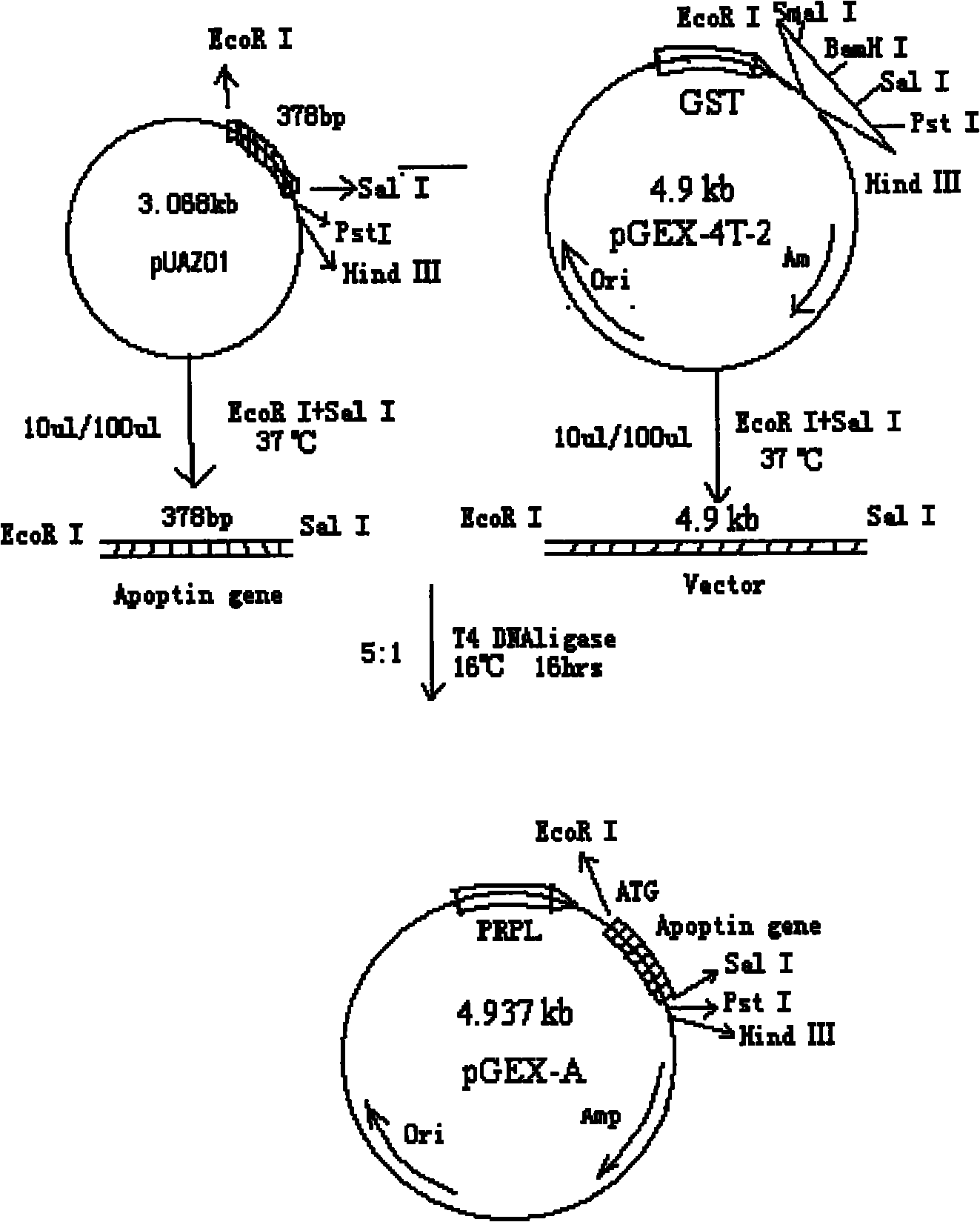
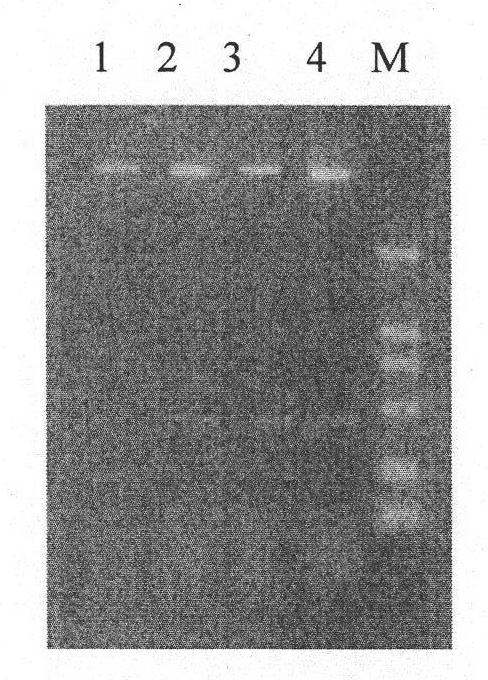
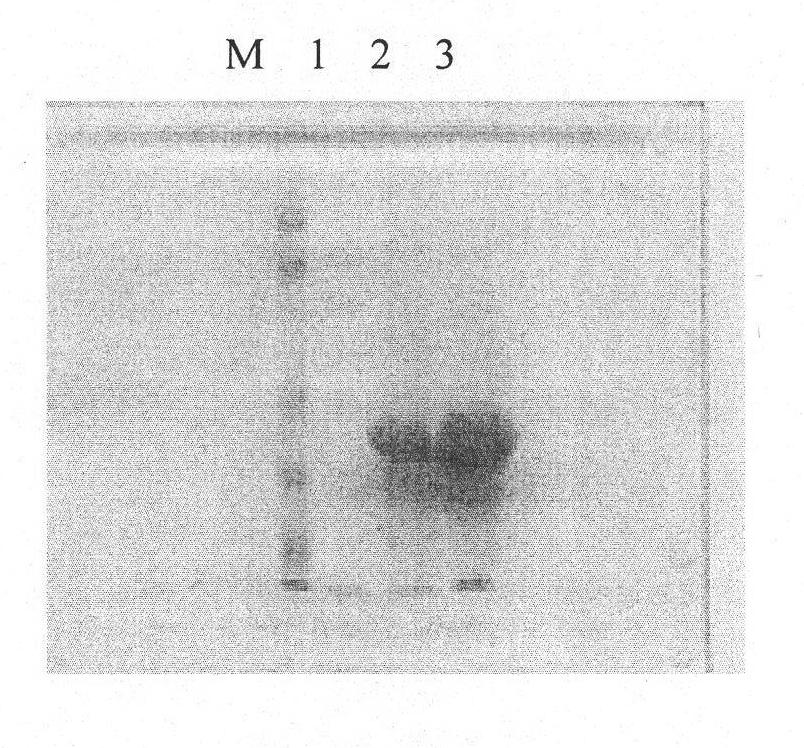
[0037] (2) Fermentation, high-efficiency expression, dissolution, renaturation and purification of GST-Apoptin fusion protein, specifically:
[0038] (2.1) Activation, fermentation and recovery of inclusion bodies of engineered bacteria Inoculate engineered strains stored at -70°C on nutrient agar plate medium containing AP, and culture at 37°C for more than 12 hours; pick a single colony and inoculate into AP-containing medium In LB liquid medium, cultivate in a shaker at 37°C for more than 12 hours, inoculate the bacterial liquid into the fermenter at a ratio of 1:10, and cultivate at 37°C; when the concentration of the bacterial liquid reaches 2.0OD, add IPTG inducer; induce Cultivate for 4 hours, and at the same time, add a small amount of filler solution continuously, and the ammonia water automatically adjusts the pH value to 7.0. The cells were collected by centrifugation, suspended in phosphate buffer (PB, pH 7.2), and subjected to high-pressure homogenization to crush...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com