Application of quinazoline compounds
A technology of quinazolines and compounds, applied in the field of medicine, can solve the problems of large drug resistance of adverse reactions, narrow use range, poor solubility of paclitaxel, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
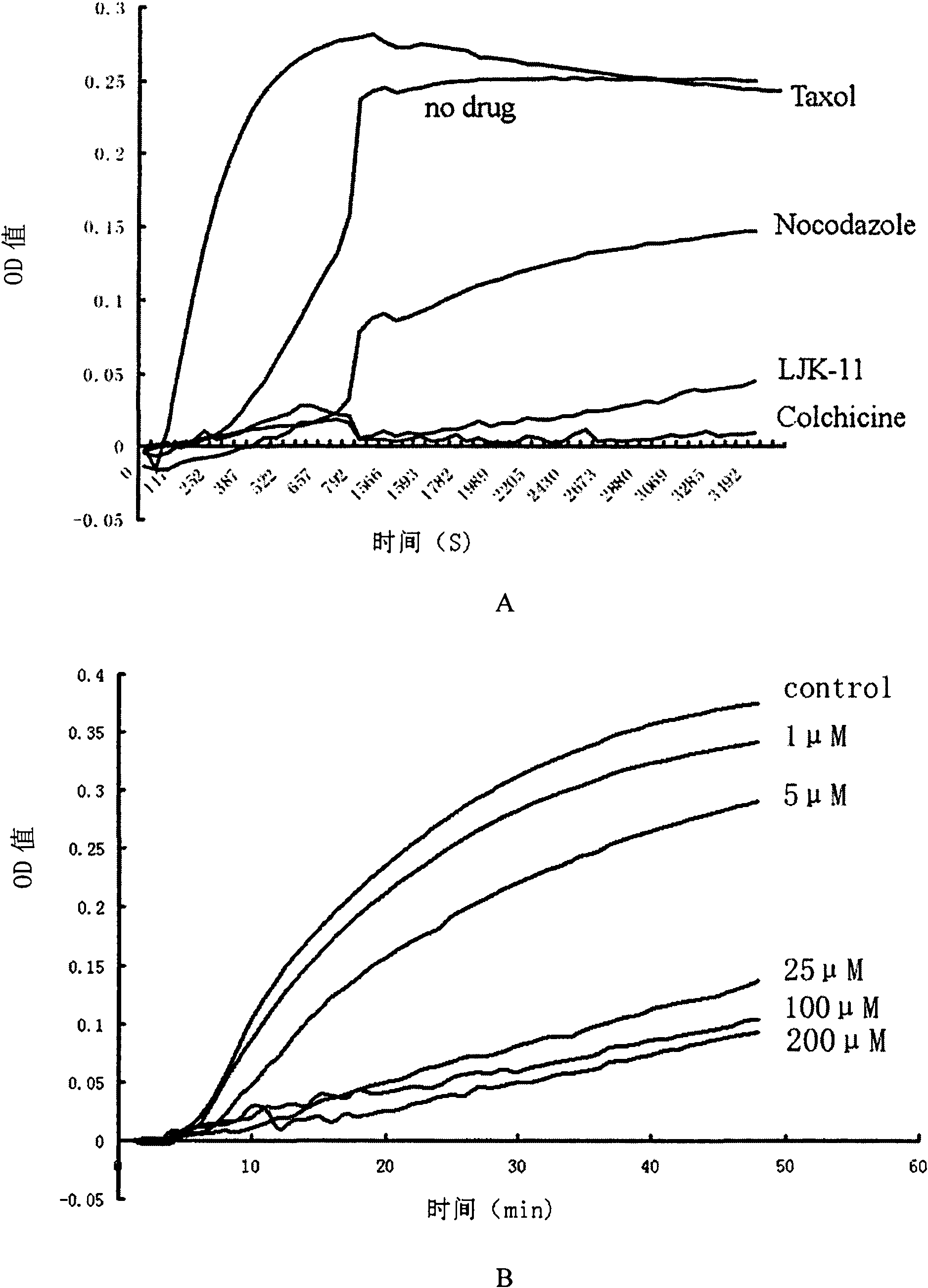
[0077] Example 1 Effect of LJK-11 on Microtubule Polymerization
[0078] Experimental instrument: Tecan GENios Pro multifunctional microplate reader
[0079] Experimental reagents: Tubulin Polymerization Assay Kit (Cat.#CDS03 and BK006)
[0080] experimental method:
[0081] 1. Preheat the 96-well plate at 37°C for 30 minutes;
[0082] 2. Pre-cool tubulin polymerization buffer at 4°C:
[0083] General Tubulin Buffer (750μl): 80mM PIPES (pH6.9), 2mM Mgcl 2 , 0.5mM EGTA;
[0084] Tubulin Glycerol Buffer (250μl): 15% glycerol in General Tubulin Buffer;
[0085] GTP Stock (200mM, 10μl): 1mM GTP;
[0086] 3. Preheat 500μl General Tubulin Buffer to room temperature;
[0087] 4. Randomly divided into 5 groups: (1) Control group: add 5 μl General Tubulin Buffer to each well; (2) LJK-11 group: add 10 μl LJK-11 to each well, the final concentrations are 200 μM, 100 μM, 25 μM, 5 μM, 1 μM, incubate at 37°C for 2 minutes; (3) Colchicine group: pipette 10 μl colchicine and dissolve ...
Embodiment 2
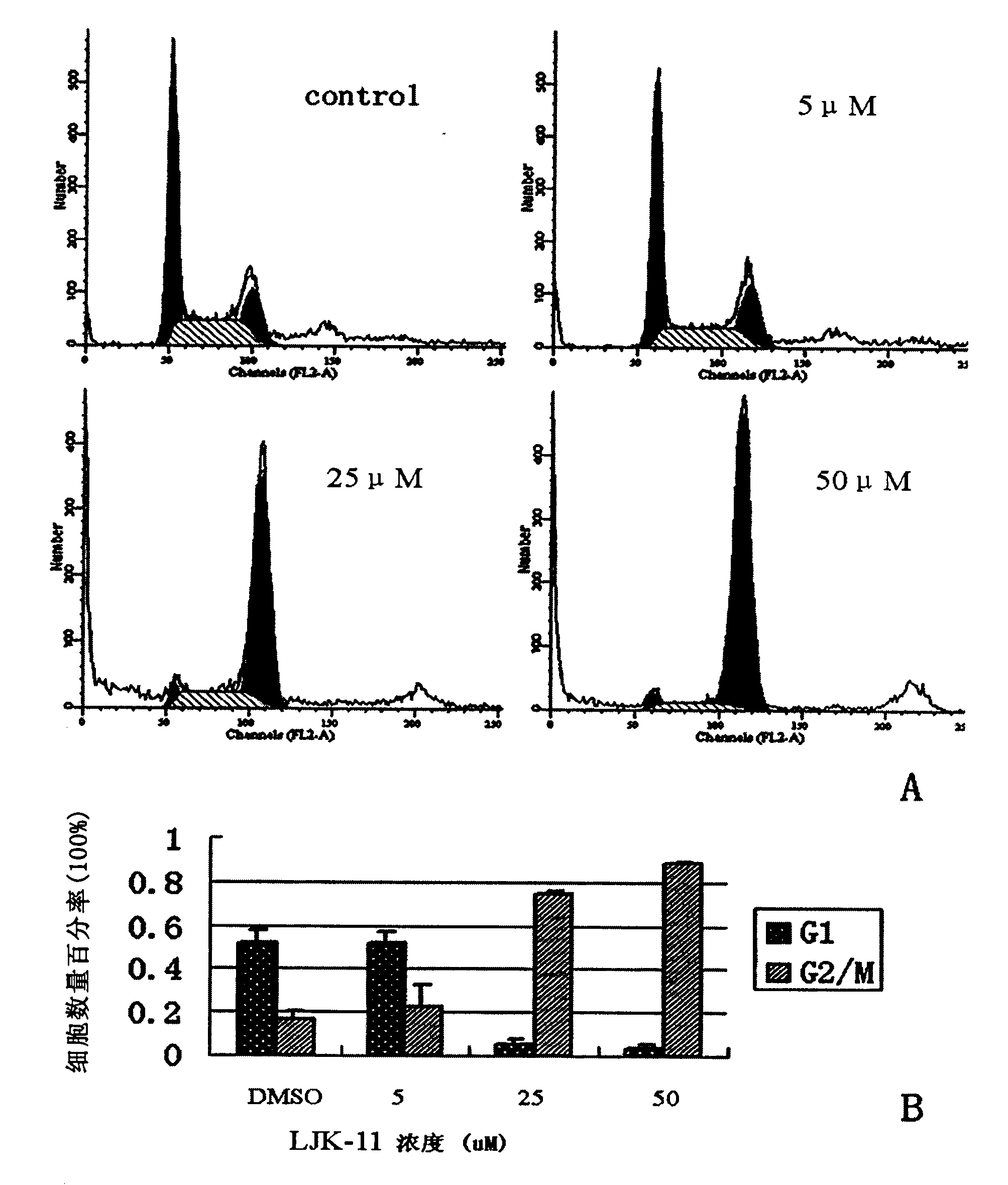
[0092] Example 2 Effect of LJK-11 on human gastric cancer HGC cell cycle
[0093] Culture HGC cells in vitro, when the growth state is good, digest and inoculate 1×10 5 Cells were placed in a 6cm culture dish and adhered to the wall overnight. After the cells were in good condition, the medium was aspirated, and LJK-11 diluted with the medium was added to the final concentrations of 5 μM, 25 μM, and 50 μM, respectively, and the medium was added to the negative control wells, and cultured at 37°C for 24 hours. Changes in cell morphology were observed under a microscope. The cells were digested with trypsin (0.25% trypsin / 0.02% EDTA), gently blown down, transferred to a centrifuge tube, centrifuged at 2000rpm for 5min, washed twice with PBS, and centrifuged at 1000rpm for 5min. Slowly add 800 μl of 75% ethanol (pre-cooled at -20°C) to the cell pellet, and shake (Vortex) while dripping to make it form a single cell as much as possible. After fixing overnight at 4°C, centrifuge...
Embodiment 3
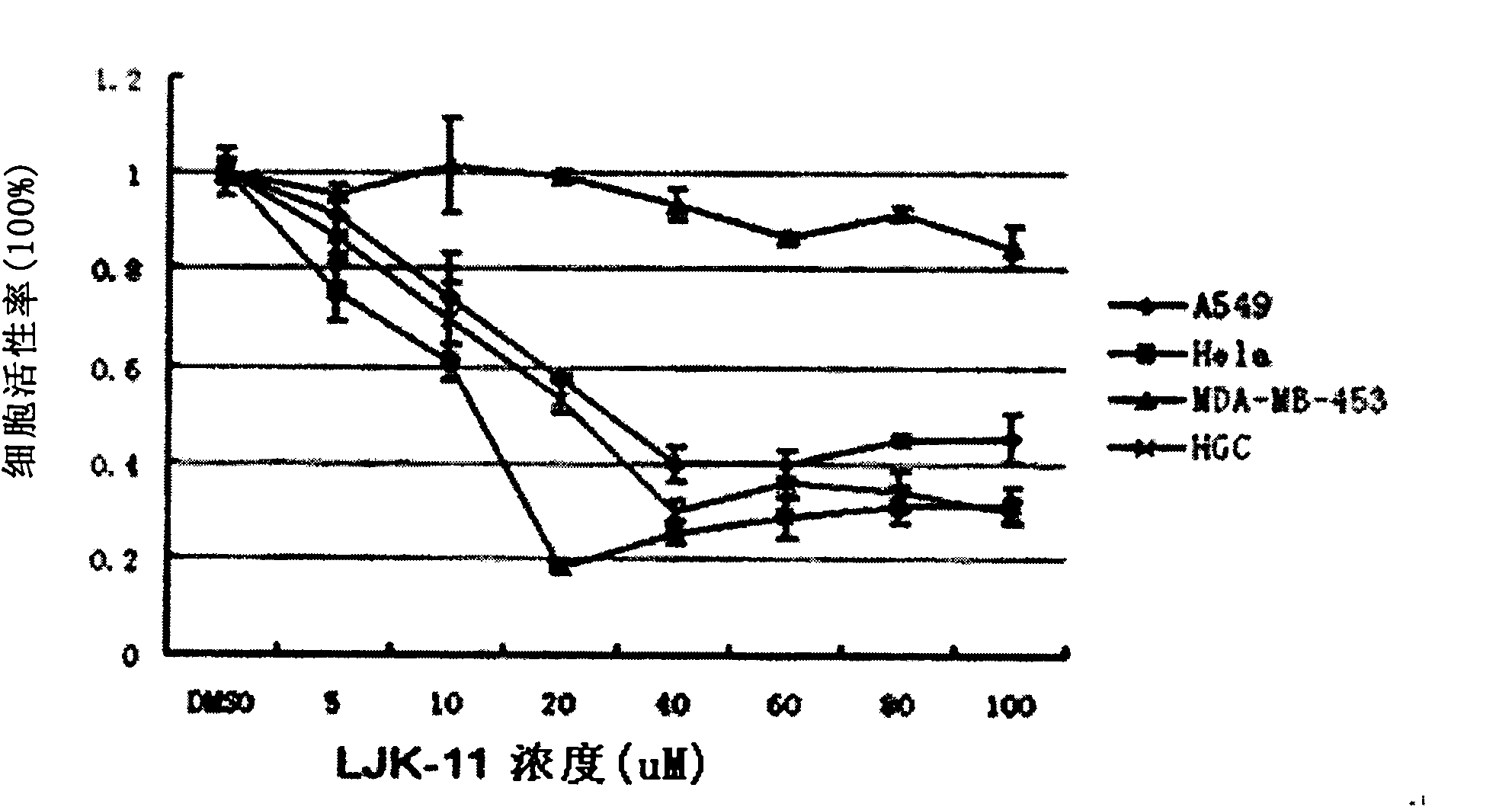
[0095] Example 3 Inhibition of Tested Drugs on the Growth of Various Tumor Cells
[0096] Human breast cancer MDA-MB-453 cells, human cervical cancer Hela cells, human lung cancer A549 cells, and human gastric cancer HGC cells were cultured in vitro. After the cells grow to the logarithmic growth phase, collect the cells, centrifuge at 1000rpm for 5 minutes, discard the supernatant, suspend with an appropriate amount of medium, and adjust the cell concentration to 3.5x10 3 / ml. The cell suspension was inoculated into a 96-well cell culture plate, 100 μl per well, placed in a cell culture incubator (37° C., 5% CO 2 ) for 24 h, and then the drug to be tested, LJK-11 (final concentrations of 1 μM, 5 μM, 10 μM, 20 μM, 40 μM, 60 μM, 80 μM, 100 μM), LJK-4 (final concentration is 40 μM), LJK-6 (final concentration is 40 μM), LJK-12 (final concentration is 40 μM), LJK-13 (final concentration is 40 μM), the negative control group was added with a final concentration of 0.5% DMSO, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com