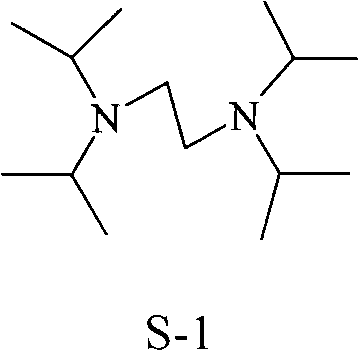
Synthesis method of N, N, N', N'-tetraisopropyl ethylene diamine
A technique of propylethylenediamine and its synthesis method, which is applied in the field of N, can solve problems such as the difficulty of solvent recovery and mechanical application, increase the difficulty of product separation, and the high price of dibromoethane, and achieve low price, reduced pressure, and simple post-treatment Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1. A method for synthesizing N,N,N',N'-tetraisopropylethylenediamine:
[0019] Add 40.5 g (0.4 mol) of diisopropylamine and 39.6 g (0.4 mol) of dichloroethane into the autoclave with a stirring temperature measuring device, and close the lid. Use N 2 After detecting leaks and replacing the air several times, the temperature was raised to 100°C and the pressure was 0.2MPa. The reaction was terminated after keeping the temperature for 4 hours.
[0020] Add NaOH aqueous solution (mass concentration 50%) to the obtained solid-liquid mixture until PH=13.5, stand still for layering, separate the organic phase (located in the upper layer) and perform intermittent distillation under reduced pressure (1266.6Pa), collect 86.5-97.0℃ From the distillate, 38.9 g of N,N,N′,N′-tetraisopropylethylenediamine was obtained, the yield was 85.2%, and the purity was 99%. The obtained product was characterized by a correct structure.
Embodiment 2
[0021] Example 2. A method for synthesizing N,N,N',N'-tetraisopropylethylenediamine:
[0022] Add 40.5g (0.4mol) of diisopropylamine and 79.2g (0.8mol) of dichloroethane into the autoclave with a stirring temperature measuring device, and close the lid of the kettle. Use N 2 After detecting leaks and replacing the air several times, the temperature was raised to 150°C and the pressure was 0.6MPa. The reaction was terminated after keeping the temperature for 6 hours.
[0023] Add KOH aqueous solution (mass concentration 50%) to the obtained solid-liquid mixture until PH=13.5, stand still for stratification, separate the organic phase (located in the upper layer) and perform intermittent distillation under reduced pressure (1266.6Pa), collect 86.5-97.0℃ From the distillate, 40.3 g of N,N,N',N'-tetraisopropylethylenediamine was obtained, the yield was 88.2%, and the purity was 99%. The resulting product was characterized by a correct structure.
Embodiment 3
[0024] Example 3. A method for synthesizing N,N,N',N'-tetraisopropylethylenediamine:
[0025] Add 40.5g (0.4mol) of diisopropylamine and 79.2g (0.8mol) of dichloroethane into the autoclave with a stirring temperature measuring device, and close the lid of the kettle. Use N 2 After detecting leaks and replacing the air several times, the temperature was raised to 140°C and the pressure was 0.5MPa. The reaction was terminated after keeping the temperature for 8 hours.
[0026] Add Na to the resulting solid-liquid mixture 2 CO 3 Aqueous solution (mass concentration 20%) until PH=11.5, stand still for stratification, separate the organic phase (located in the upper layer) and perform intermittent distillation under reduced pressure (1266.6Pa), collect fractions at 86.5-97.0°C to obtain products N, N, N ',N'-tetraisopropylethylenediamine 40.2g, the yield was 88.0%, the purity was 99%, and the resulting product was characterized by the correct structure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com