Method for extracting lithium from salt lake brine by adsorption method
A salt lake brine and adsorption technology, applied in the field of lithium extraction, can solve the problems of low lithium ion adsorption efficiency and high production cost, and achieve the effects of simple preparation method, low price and improved adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation method of aluminum-containing salt type adsorption resin is as follows:
[0041] (1) Insert LiCl into Al(OH) 3 The reaction produces the insertion formula LiCl·2Al(OH) 3 ·nH 2 O compound;
[0042](2) Use the fluororesin high molecular polymer as the binder, dissolve it in the volatile organic solvent toluene at a weight ratio of 1:3, and then add the plug-in LiCl 2Al(OH ) 3 ·nH 2 O compound, then pelletized and the solvent removed to produce an aluminum salt-type adsorption resin.
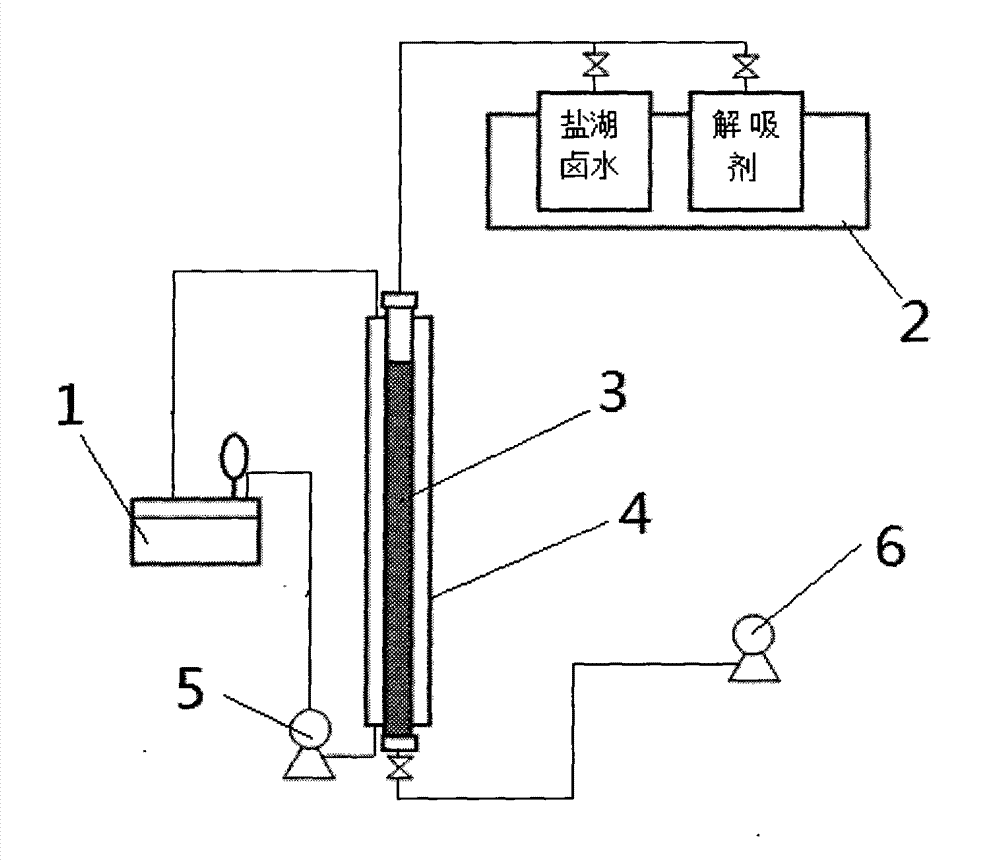
[0043] Adsorbent filling: take 500ml of the above-mentioned aluminum-containing salt-type adsorbent resin and put it into the measuring cylinder 3 (denoted as 1BV), and then put the measuring cylinder 3 into the stainless steel column 4 with a jacket of Φ30*1000mm;
[0044] Adsorbent pretreatment: take 10BV deionized water, wash with 4BV / H until the conductance of the water out of the detection outlet is between 20-30us.
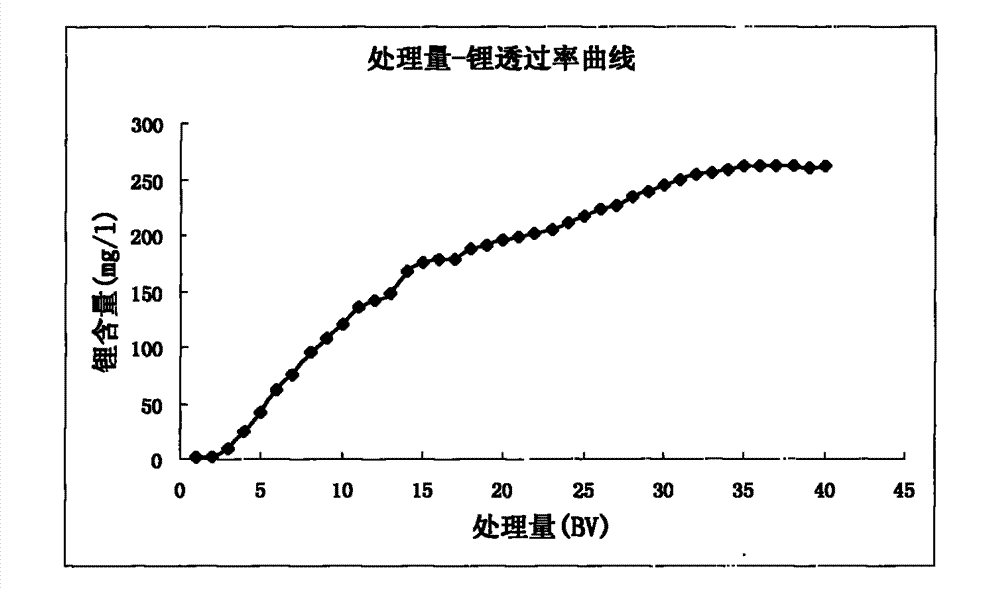
[0045] The method of extracting lithium from s...
Embodiment 2
[0060] The preparation method of aluminum-containing salt type adsorption resin is as follows:
[0061] (1) Insert LiCl into Al(OH) 3 The reaction produces the insertion formula LiCl·2Al(OH) 3 ·nH 2 O compound;
[0062] (2) Polyvinyl chloride macromolecular polymer is used as binder, and is dissolved in easily volatile organic solvent benzene by weight ratio 1: 15, then adds plug-in type LiCl 2Al ( Oh) 3 ·nH 2 O compound, then pelletized and the solvent removed to produce an aluminum salt-type adsorption resin.
[0063] The temperature control in the loading of adsorbent, adsorbent pretreatment and adsorption process is the same as embodiment 1;
[0064] The brine for adsorption contains Li: 250-300mg / l, Mg: 200-300g / l, density 1.13g / l, Baume degree 33-35, PH: 6.8.
[0065] (1) Adsorb lithium ions in salt lake brine with adsorption resin
[0066] Temperature control: The feed liquid is heated by a water bath to maintain a constant temperature, and the jacket uses a sel...
Embodiment 3-10
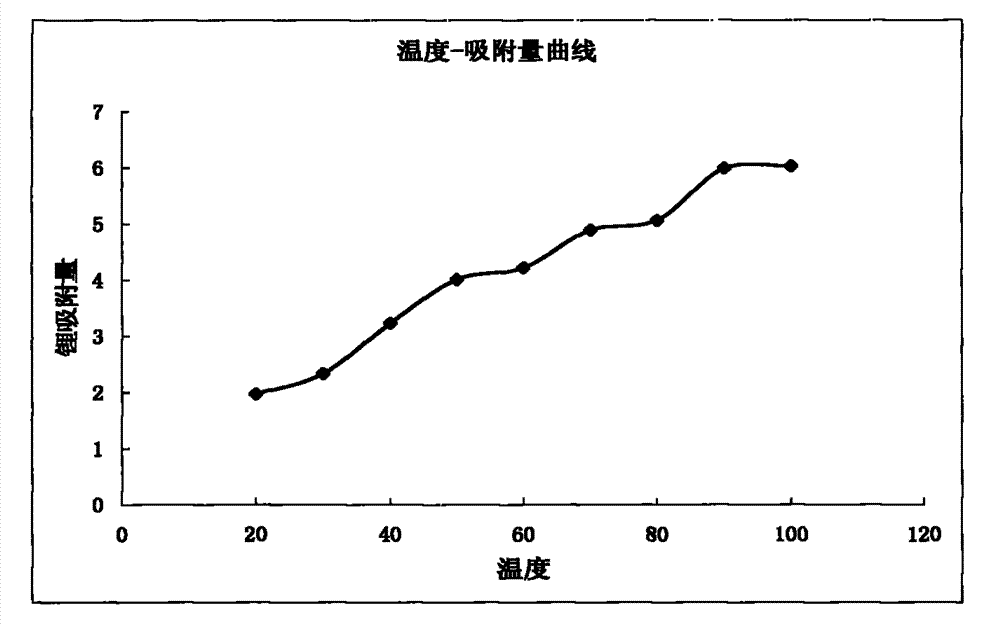
[0078] Except that temperature conditions and flow rates are different, all the other steps and conditions are the same as in Example 2. The specific temperature, flow rates and yields are shown in Table 1.
[0079] Table 1
[0080] Example serial number
temperature(℃)
Velocity(BV / H)
Adsorption capacity
Lithium yield
Example 2
20
5
1.98
59.3%
[0081] Example 3
30
5
2.53
61.7%
Example 4
40
5
3.02
73.6%
Example 5
50
5
3.11
74.9%
Example 6
60
5
3.87
78.7%
Example 7
70
5
4.02
90.1%
Example 8
80
5
4.48
91.9%
Example 9
90
5
4.53
95.8%
Example 10
100
5
4.71
98.7%
[0082] The temperature-adsorption capacity curve of embodiment 2-embodiment 10 sees figure 2 ,Depend on...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com