Novel tumor marker
A tumor and conjugate technology, applied in the field of tumor treatment and tumor metastasis, can solve the problems of unidentified Hsp90α existence form, no mention of tumor metastasis correlation, no definition of normal and abnormal content ranges, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
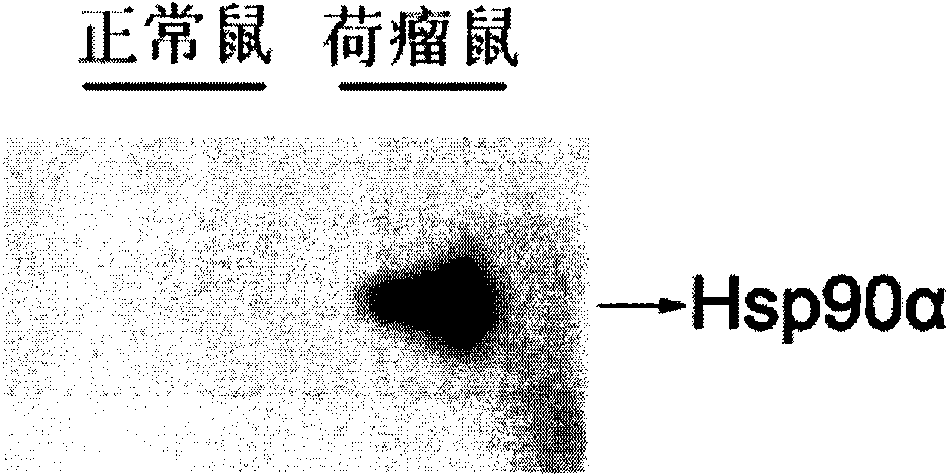
[0112] Example 1: Collection and preparation of mouse plasma samples, and detection of plasma Hsp90α
[0113] Select Balb / c mice with an average body weight of about 20 grams (purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.), and randomly divide them into two groups, with 3 mice in each group, and H22 mouse liver cancer cells were implanted in the axilla of one group (CCTCC, number: GDC091) 10 cells per access 6 A control group was not inoculated with tumors. When the diameter of the mouse tumor grew to an average of 2 cm (about 20 days), blood was collected from the fundus venous plexus, and an anticoagulant was added to the blood to avoid hemolysis. Samples were recollected if hemolyzed. After the whole blood was collected, it was centrifuged twice at 4°C and 6000g, and the supernatant was taken, and the content of Hsp90α in the plasma was detected by Western blotting. The antibody was Rabbit anti-human Hsp90αpAb (Labvasion). The BCA method (Pi...
Embodiment 2
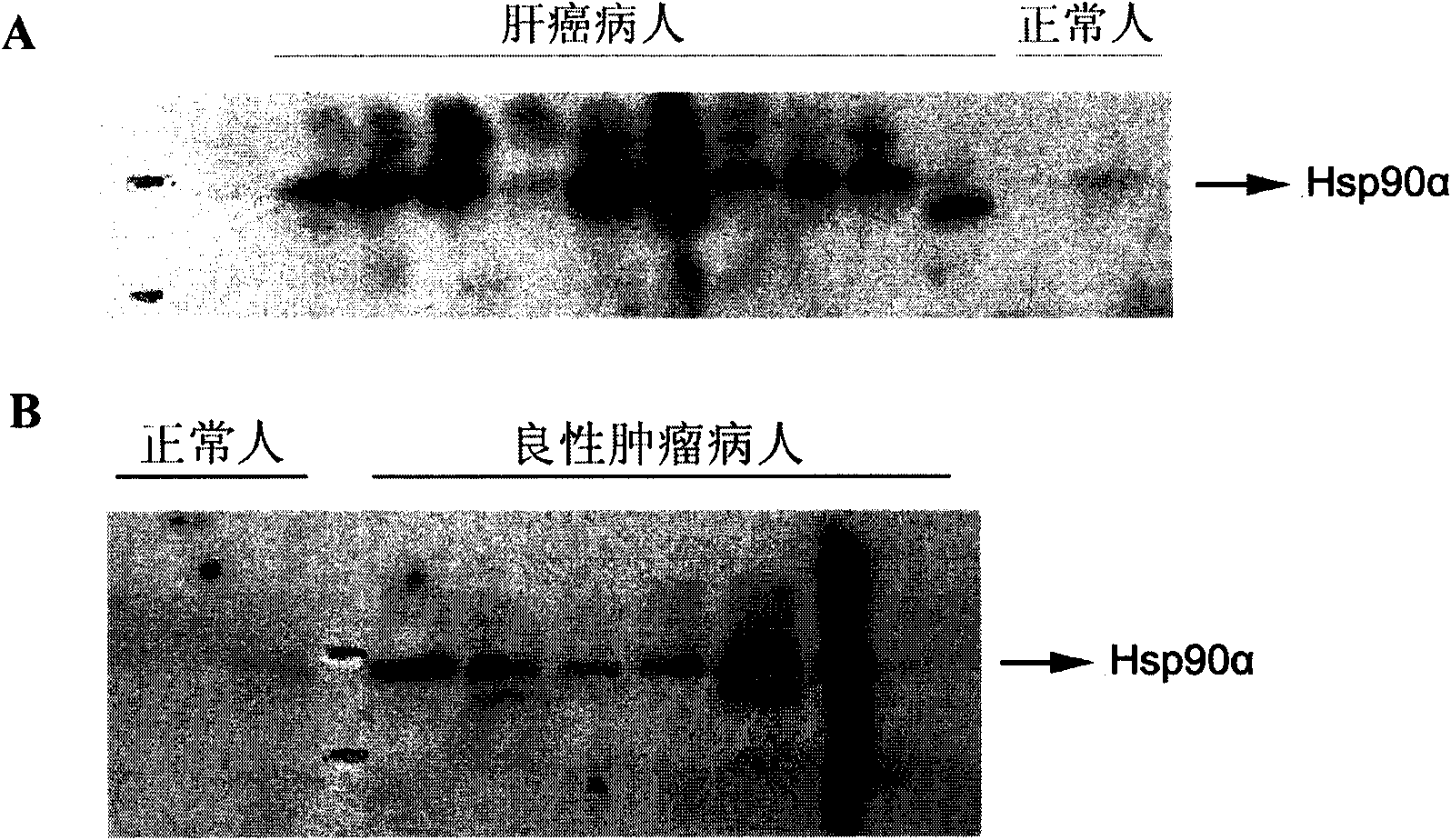
[0114] Example 2: Collection and preparation of plasma samples from normal people and tumor patients, and detection of plasma Hsp90α
[0115] Take whole blood from normal people or cancer patients and send it to the laboratory within 24 hours under low temperature conditions (about 4°C). Hemolysis must be avoided. If the sample is hemolyzed, it must be collected again. Centrifuge twice at 4°C and 6000g, take the supernatant, and detect the content of Hsp90α in plasma by Western blotting. If it cannot be detected immediately, it will be aliquoted and stored at -80°C. The test results will be compared with the clinical diagnosis to verify the correlation between the Hsp90α content in plasma and the degree of tumor malignancy.
[0116] The specific operation method of Western blot detection is: the plasma sample is mixed with the loading buffer 1:1, and 1-2 microliters of the sample are loaded for SDS-PAGE. The primary antibody is a specific antibody that recognizes plasma Hsp90α...
Embodiment 3
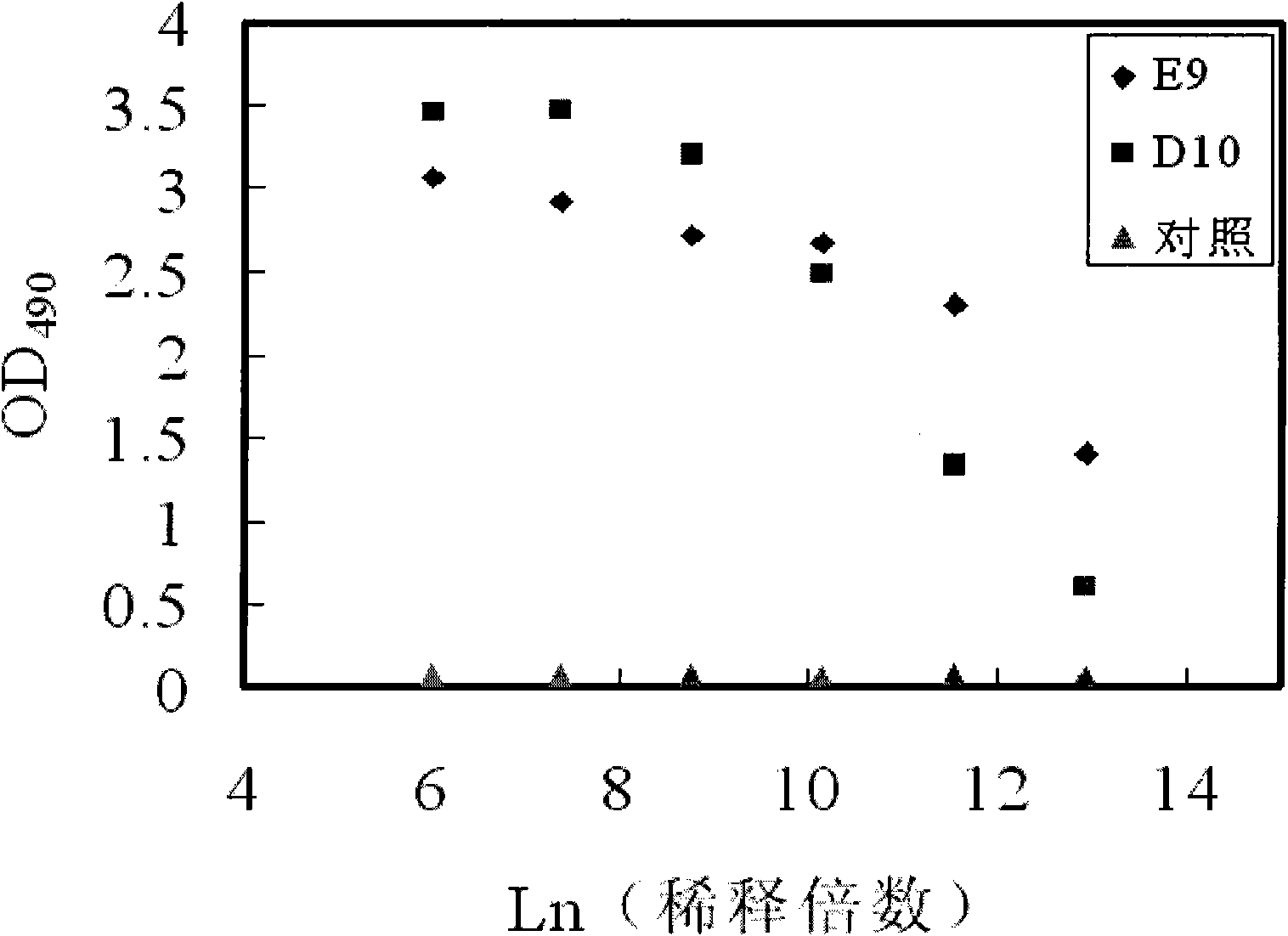
[0117] Example 3: Preparation of Hsp90α-specific rabbit polyclonal antibody and mouse monoclonal antibody
[0118] Primers (synthesized from Invitrogen) and Pfu DNA polymerase ( Source: NEB) The full-length sequence of Hsp90α was amplified from a human liver cDNA library (source: Stratagene), and the fragment was double digested with Sph1 and Sal1 (source: NEB) and the pQE80L vector (source: Qiagen) to obtain the fragment Ligation was performed using T4 ligase (source: NEB). The ligation product was transformed into Top10 Escherichia coli competent cells (source: Transgen) for amplification and verification, and the verified plasmid was then transformed into BL21DE3 Escherichia coli competent cells (source: Transgen) for expression to obtain recombinant human Hsp90α protein. Purification method of recombinant human Hsp90α protein: ion exchange chromatography SP Hp, pH 6.8, collect the elution peak with a conductance of 10ms / ml; Q HP, pH 7.8, collect the elution peak with a co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com