Production method of dalteparin sodium
A technology of darteparin and its production method, which is applied in the field of preparation of biochemical drugs, can solve the problems of poor product stability, high product yield, unqualified structure, etc., and achieve the effect of good stability and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
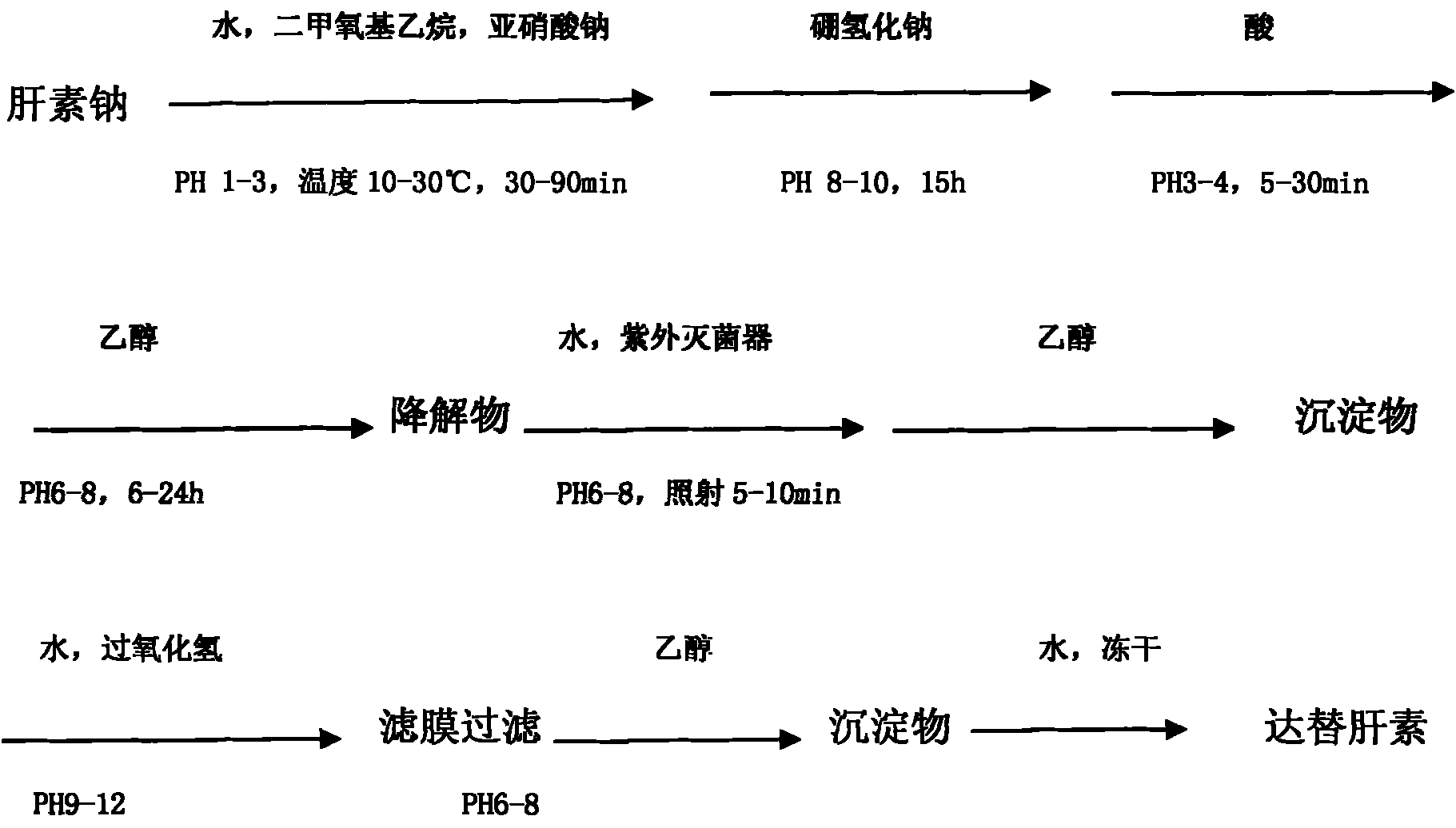
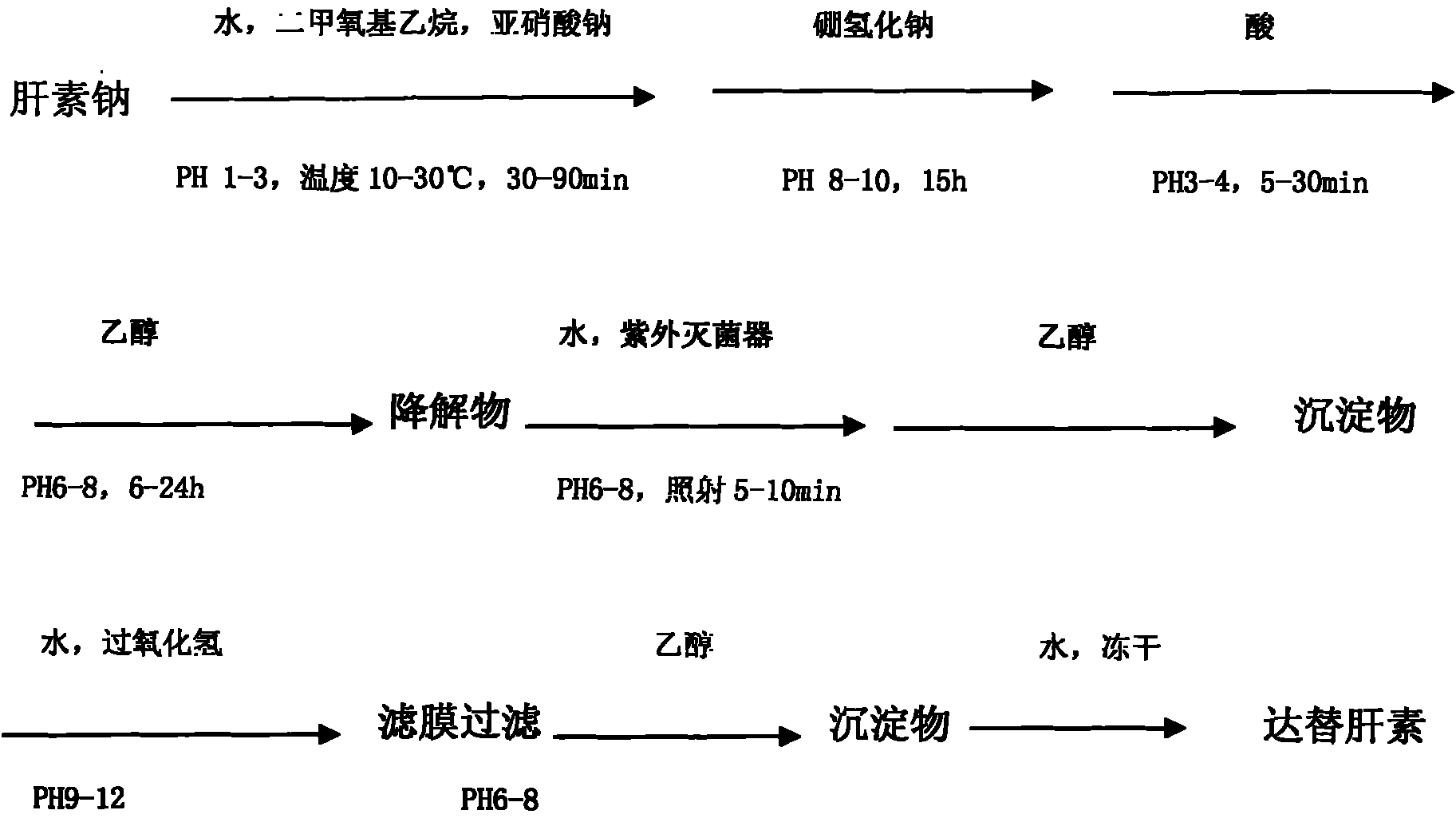
[0014] Example 1: Take high-quality heparin sodium, add purified water to dissolve it to a concentration of 3% to 10%, adjust the pH to 1.0 to 3.0 with 2 to 6 mol / L hydrochloric acid, and then add 0.5 to 2.0 times the volume of dimethoxyethyl ether alkanes, adjust the temperature to 10-30°C, add 1.0%-3.0% sodium nitrite, stir for 30-90 minutes, and control the temperature at 10-30°C; after the degradation is completed, adjust the pH to 8-10, and then add 0.5% ~2% sodium borohydride, stirred for 15 hours. Then adjust the pH to 3.0-4.0 with hydrochloric acid, adjust the pH to 6-8 after reacting for 5-30 minutes, add 1.5-3 times the amount of 92% ethanol for precipitation, and place it for 6-24 hours. Add purified water to dissolve the obtained precipitate to a concentration of 5% to 20%, adjust the pH to 6 to 8, filter with a 0.65um filter membrane, put the filtrate into a UV sterilizer and irradiate it for 5 to 10 minutes, add 1.5 to 3 times the amount of 92% ethanol to precipi...
Embodiment 2
[0015] Example 2: Dalteparin is achieved through the following process, adding heparin sodium into purified water to dissolve to a concentration of 3% to 10%, adjusting the pH to 1.0 to 3.0 with 4mol / L hydrochloric acid, and then adding 0.5 to 2.0 times the volume of the solution Dimethoxyethane, adjust the temperature to 10-30°C, add 1.0%-3% sodium nitrite, stir for 30-90 minutes, and control the temperature at 10-30°C; after the degradation is completed, adjust the pH to 8-10 , Then add 0.5% ~ 2% sodium borohydride, and stir the reaction for 15h. Then adjust the pH to 3.0-4.0 with hydrochloric acid, adjust the pH to 6-8 after reacting for 5-30 minutes, add 1.5-3 times the concentration of 90%-95% ethanol for precipitation, and place it for 6-24 hours. Add the obtained precipitate into purified water to dissolve to a concentration of 5% to 20%, adjust the pH to 6 to 8, filter with a 0.65um filter membrane, put the filtrate into a UV sterilizer and irradiate it for 5 to 10 min...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com