Method for separating and purifying human amniotic fluid-derived mesenchymal stemcells
A technology for the separation and purification of mesenchymal stem cells, applied in the field of separation and purification of amniotic fluid mesenchymal stem cells, can solve the problems of high cost and complicated operation, and achieve the effect of convenient raw material acquisition, simple acquisition method and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Isolation, purification and expansion of human amniotic fluid stem cells in the discarded supernatant of amniotic fluid culture for prenatal diagnosis
[0024] (1) Aseptically take mid-term amniotic fluid samples. The amniotic fluid comes from outpatients in the prenatal diagnosis clinic of the Obstetrics and Gynecology Hospital Affiliated to Zhejiang University. With my consent, the discarded supernatant is used for related research. 12ml of mid-term amniotic fluid samples are centrifuged at 1500rpm for 10min; Clear, add 1m cell culture medium II, resuspend the cell pellet, transfer to T25 culture flask for culture. After 7 days, the adherent cells were harvested for prenatal diagnosis, and the discarded supernatant was collected for subsequent use; the final concentration of the cell culture medium II was composed as follows: 80% (v / v) low-sugar DMEM+20% (v / v) FBS+ 4ng / ml bFGF+100U / ml penicillin+100U / ml streptomycin.
[0025] (2) Step (1) Discard the super...
Embodiment 2
[0027] Example 2: Detection of Surface Antigen Characteristics, Immunogenicity and Totipotent Stem Cell Markers
[0028] The specific analysis method is as follows:
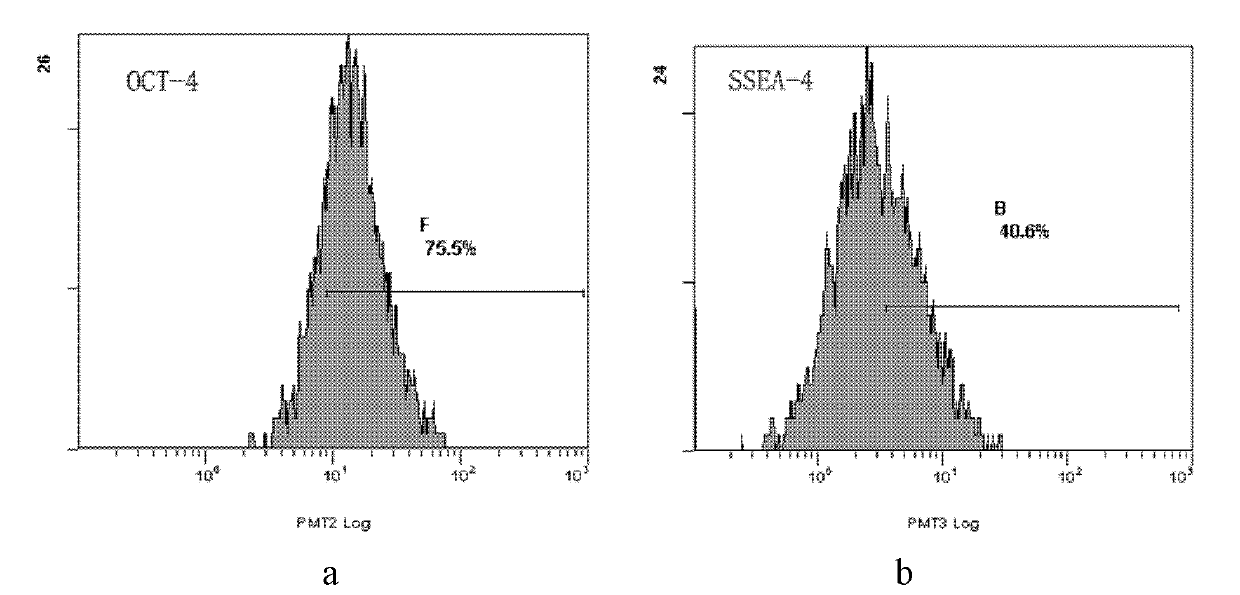
[0029] 1. Antigen characteristics and immunogenicity detection
[0030] (1) Digest the cells obtained in Example 1 with trypsin, add an equal amount of culture medium III, and terminate the digestion. The composition of the final concentration of the cell culture medium III is as follows: 85% (v / v) low-sugar DMEM+10% (v / v) FBS+100U / ml penicillin+100U / ml streptomycin;
[0031](2) After washing once with 10ml of PBS, resuspend in 1.2ml of PBS containing 1% (v / v) FBS to prepare a cell suspension, divide into 8 tubes according to the amount of 100 μL per tube, and control the amount of cells in each tube at 5×10 5 ~1×10 6 indivual;
[0032] (3) Add 20 μL each of CD29 and its isotype control, CD34 and its isotype control, CD105 and its isotype control, HLA-DR and its isotype control in sequence, and incubate at 4...
Embodiment 3
[0043] Example 3: Identification of multi-lineage differentiation potential
[0044] 1. Induced differentiation into adipocytes
[0045] Induction medium composition: low-sugar DMEM+15% (v / v) FBS+fat additive (1umol / L dexamethasone+10mg / L insulin+0.5mmol / L isobutylmethylxanthine+200umol / L indomethacin)
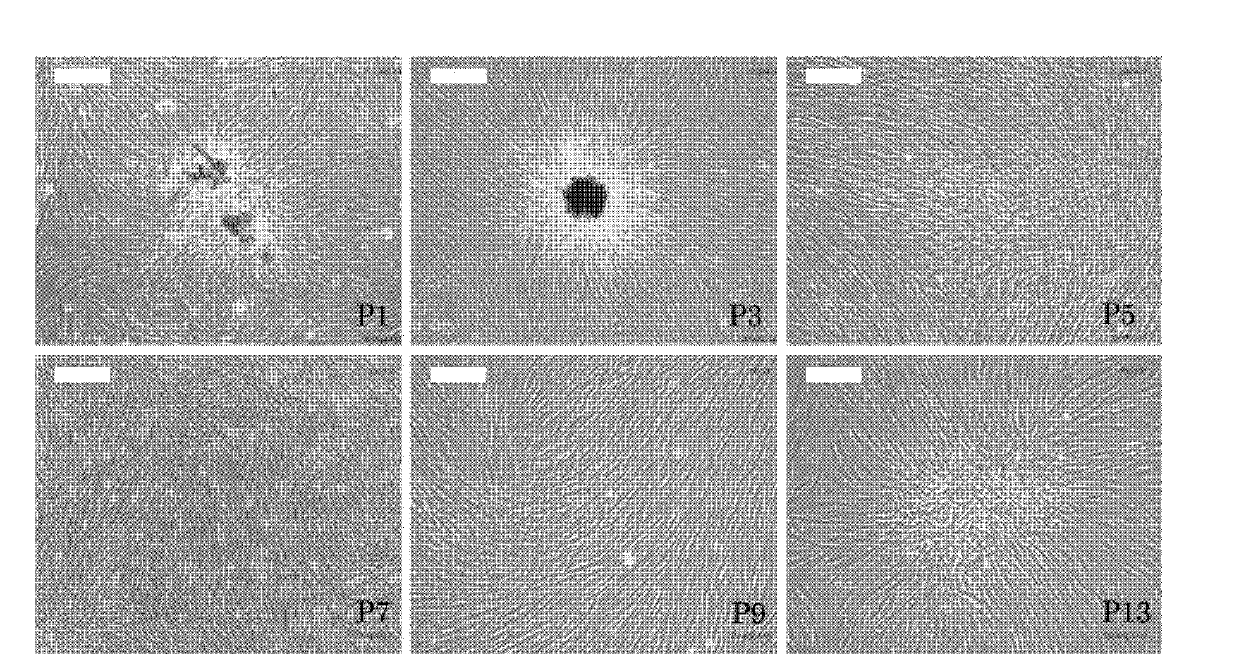
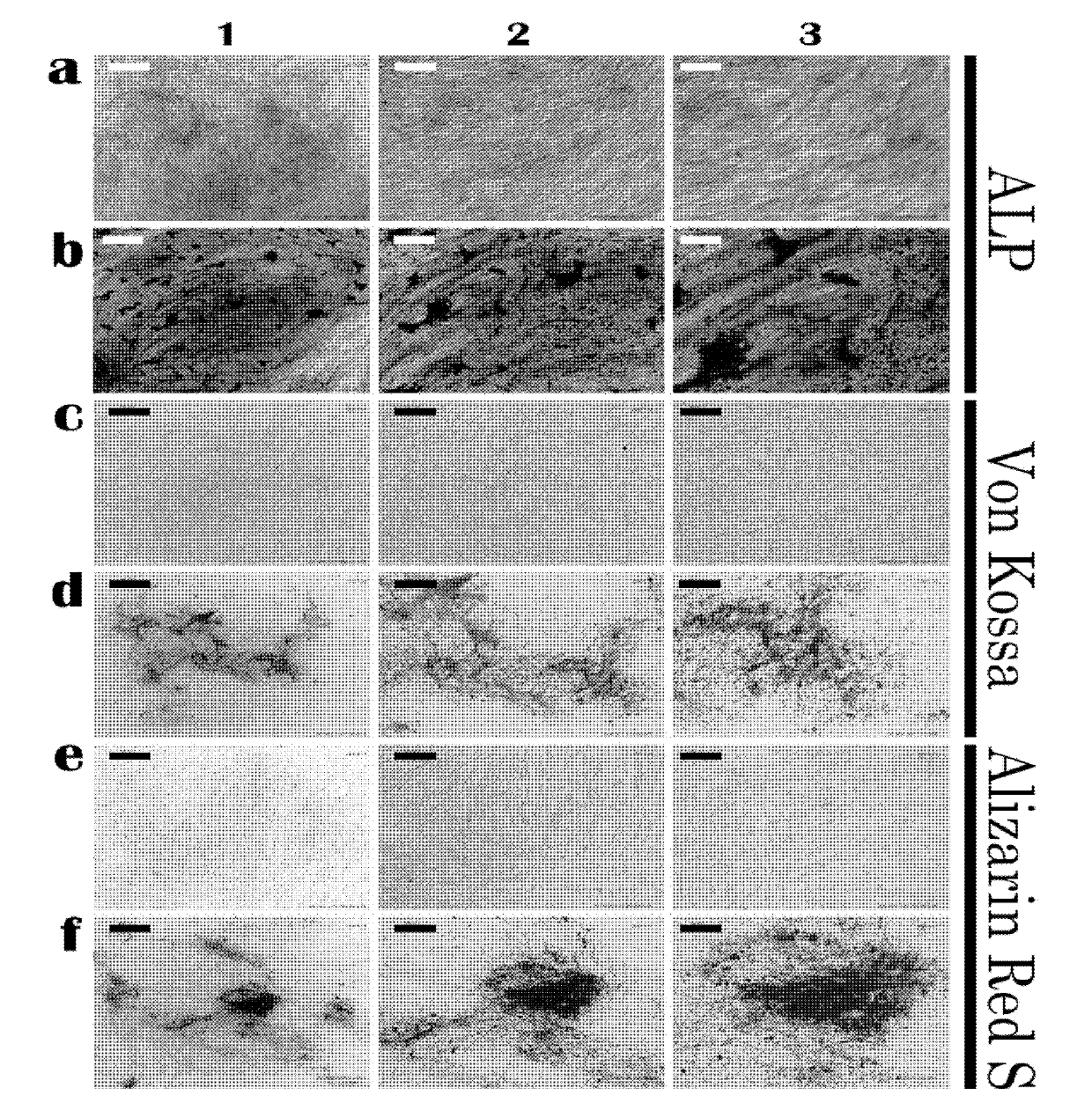
[0046] Induction method: Human amniotic fluid stem cells obtained in Example 1 were mixed with 3-5×10 3 piece / cm 2 The cell density was seeded in 24-well plates, and when 90% confluence was reached, it was replaced with adipose induction medium. The medium was changed every 3 to 4 days, and after 2 weeks of induction, positively stained fat particles were observed by Oil Red O staining ( Figure 4 ), and by RT-PCR (detection primers Forward: 5'-GCCATCCGCATCTTTCGA-3', Reverse: 5'-AGGACTCAGGGTGGTTCA-3'), the transcription factor peroxidase proliferator-activated receptor highly expressed in adipose tissue can be detected The expression of γ (PPARγ) indicates that human amnio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com