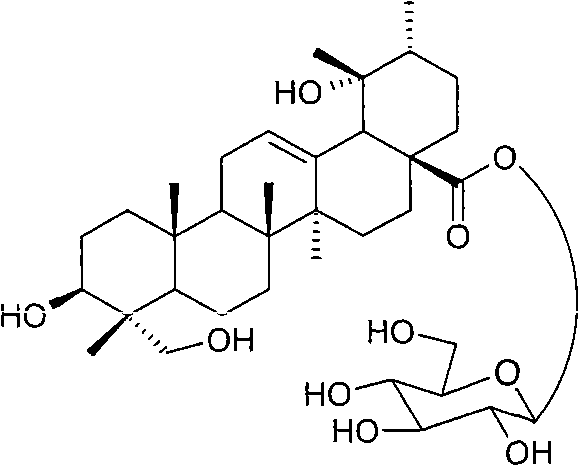
Application of pedunculoside in preparing medicine for treating coronary heart disease
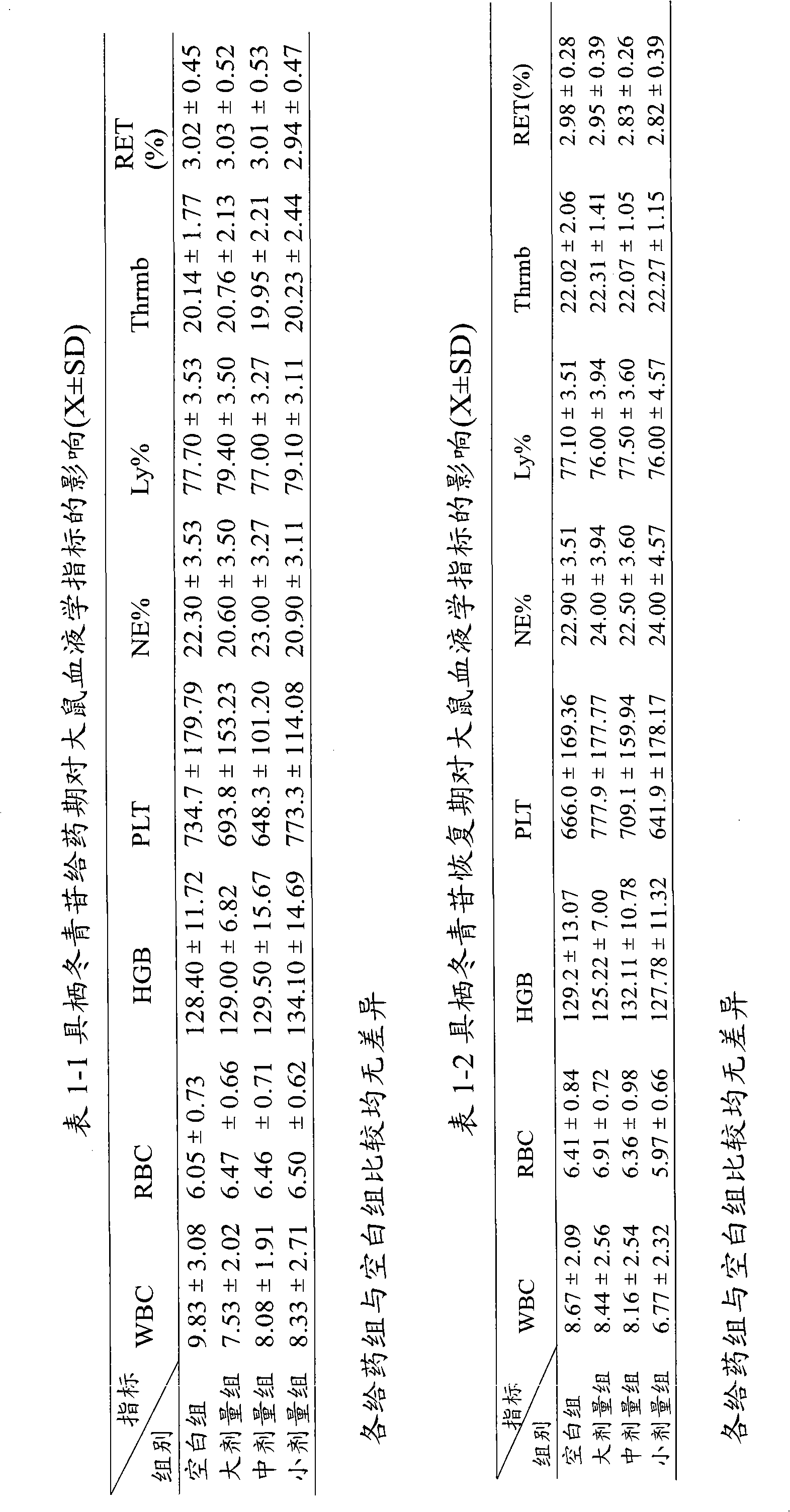
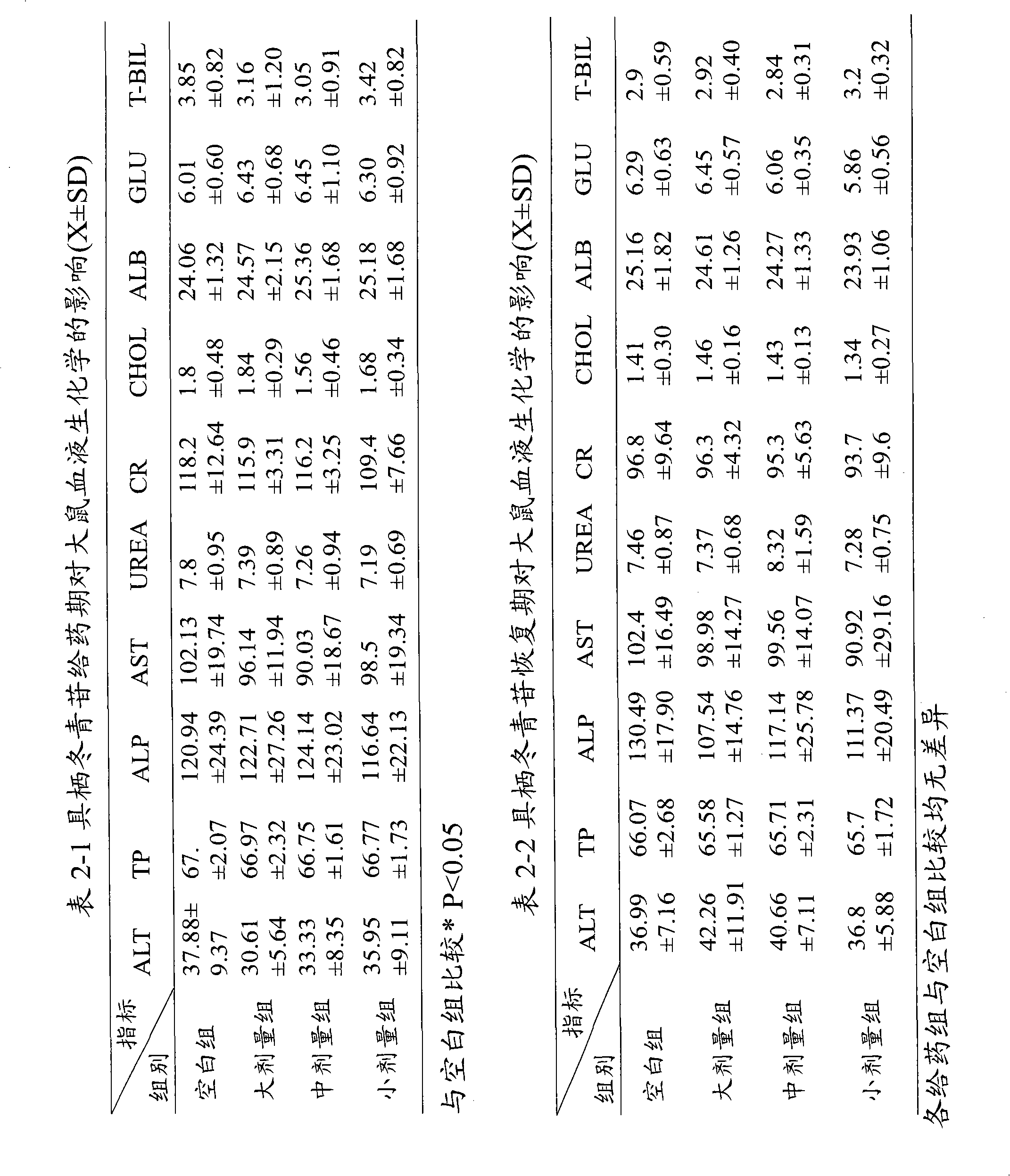
A technology with holothrin and coronary heart disease, which is applied in the field of medicine for cardiovascular system diseases, can solve the problems that the process conditions cannot meet the needs of industrialized production, the operation is complicated, and the cycle is long, so as to reduce the area of cerebral infarction and reduce the myocardial oxygen consumption index. , a significant effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Rescue 2000 g of Bing Bing coarse powder, extract three times with 6 times of 80% ethanol, extract for 2, 1, and 1 hours respectively, filter, combine the filtrates, concentrate to extract, add 6000 ml of water, heat in a water bath, and stir , stand overnight, filter to get the precipitate and filtrate, add 50-95% ethanol to reflux for extraction, filter, concentrate the filtrate, place, filter, and dry the precipitate to obtain total saponins, add 10-100 times the total saponins The 50-95% ethanol crystallizes to obtain a part of staphyloside. The filtrate was concentrated to 1000ml, allowed to cool to room temperature, filtered, the precipitate was washed three times with an appropriate amount of water, dried, crystallized with 10-50 times the amount of 50-95% ethanol, and another part of the crude crystals of staphyloside was obtained, twice. The obtained tintosides are combined, and recrystallized for several times with 10-100 times the amount of 50-95% ...
Embodiment 2
[0024] Take the aspartoside prepared by the method of Example 1, and adopt the preparation method of the infusion solution known in the art to prepare the aspartate sodium chloride injection and the aspartate glucose injection: the aspartate 40mg, Sodium Chloride 2.25g is made into Peltatin Sodium Chloride Injection, its specification is 500ml, intravenous injection, 1 bottle at a time, once a day; Peltatin 40mg, Glucose 12.5g, made of Peltatin Glycoside Glucose Injection, its specification is 500ml, intravenous injection, 1 bottle each time, once a day.
Embodiment 3
[0025] Example 3: Take 20 g of tintoside prepared according to the method of Example 1, add an appropriate amount of water for injection, adjust the pH value to 7 with sodium carbonate, stir to dissolve, and add an amount equivalent to 10-15% of tintoside. The mannitol for injection is sterilized and filtered, the content is determined, 1000 pieces are divided into 20 mg / piece, and the seal is sealed. Intravenous injection, 40 mg each time, once a day.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum tolerated dose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com