Quality analysis method of compound Ganmaoling tablets
A technology for quality analysis of Ganmaoling tablets, applied in the field of quality analysis of compound Ganmaoling tablets, can solve the problems of high detection cost, heavy workload, long detection time, etc., and achieve the effects of high sensitivity, improved work efficiency, and stable baseline.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
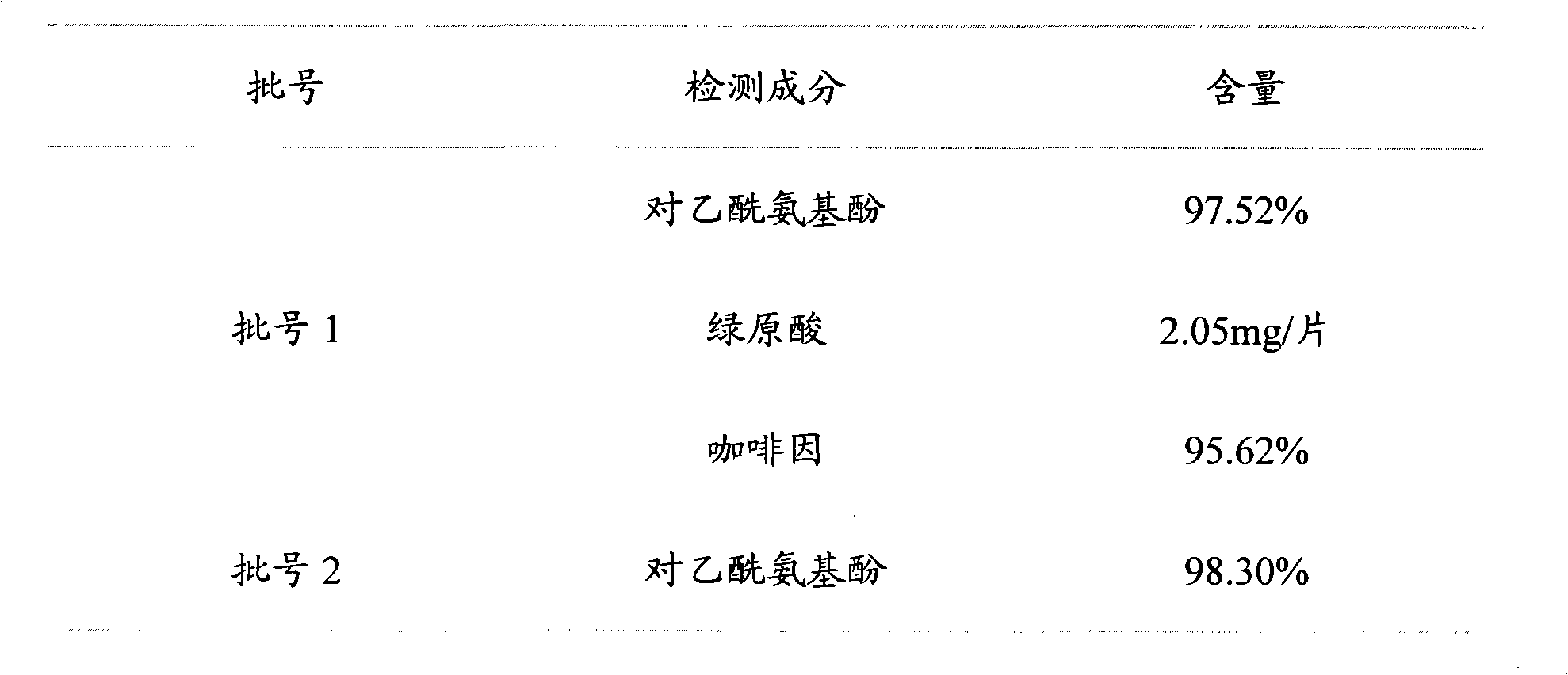
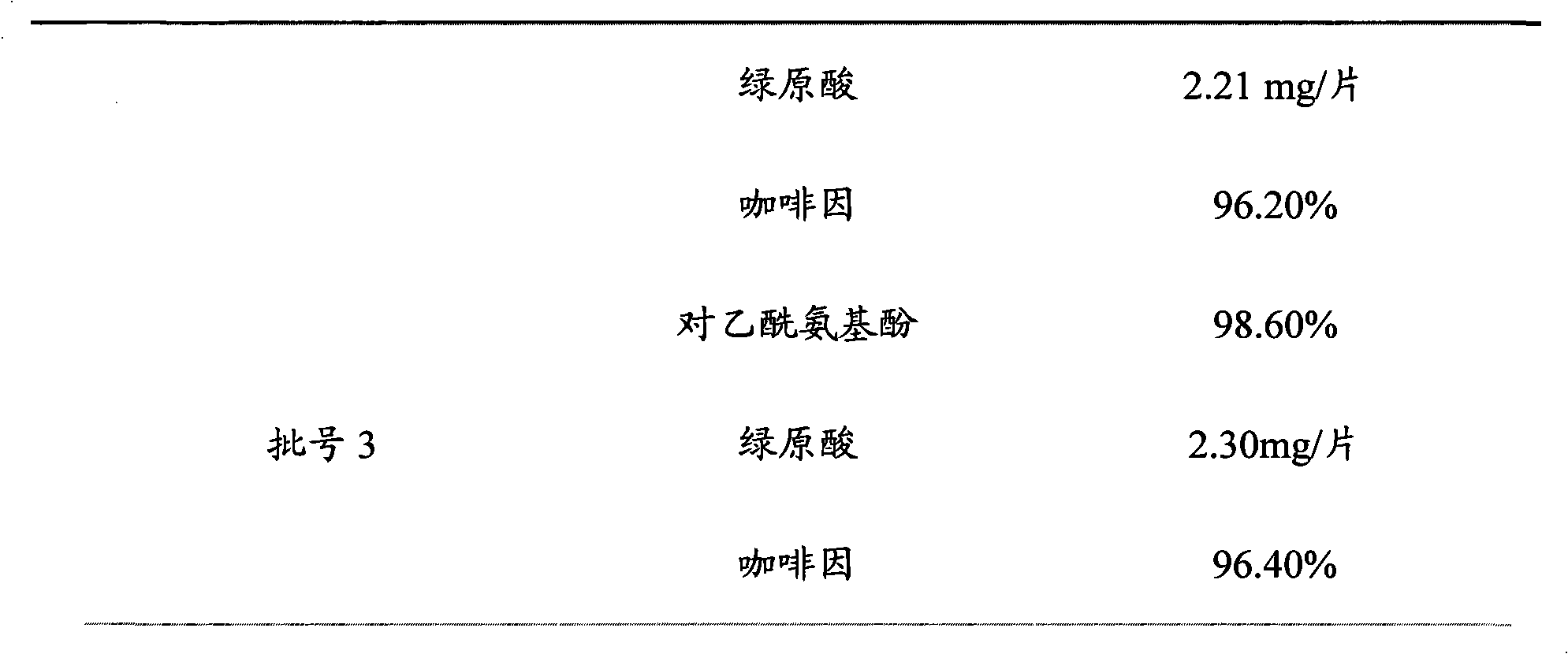
[0020] Embodiment 1 A kind of quality analysis method of Compound Ganmaoling Tablets
[0021] The content of chlorogenic acid, acetaminophen and caffeine in Compound Ganmaoling Tablets was determined by HLPC, including the following steps:
[0022] (1) Prepare the test solution
[0023] Take 10 tablets of Compound Ganmaoling Tablets, remove the coating, accurately weigh, grind into fine powder, accurately weigh about 0.67g of the fine powder, put it in a stoppered Erlenmeyer flask, add methanol-0.4% phosphoric acid with a volume ratio of 12:88 Solution 25ml, accurately weighed, after ultrasonic vibration for 20 minutes, take it out and let it cool, then accurately weigh the weight, make up the lost weight with methanol-0.4% phosphoric acid solution with a volume ratio of 12:88, shake well, filter, Precisely draw 2ml of the filtrate, put it in a 10ml brown measuring bottle, add methanol-0.4% phosphoric acid solution with a volume ratio of 12:88 to dilute to the mark, shake wel...
Embodiment 2
[0033] Example 2 Conditional screening and methodological verification of a quality analysis method for Compound Ganmaoling Tablets
[0034] 1. Preparation of test article
[0035] Refer to the relevant literature and use water ultrasonic extraction, and there are many impurities. Using methanol as solvent, the chromatographic peak shape is poor. After dissolving with an appropriate amount of methanol, and then diluting to volume with phosphate buffer, the resolution of paracetamol, chlorogenic acid and caffeine chromatographic peaks and adjacent peaks is greater than 1.5, the purity is better, and the content is the highest. Therefore, this preparation method was chosen.
[0036] 2. Measurement wavelength
[0037] Prepare acetaminophen, chlorogenic acid and caffeine reference substance solution of appropriate concentration, precisely draw 20 μl of the reference substance solution and inject it into the liquid chromatograph, check the peak spectrum of paracetamol, chlorogen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com