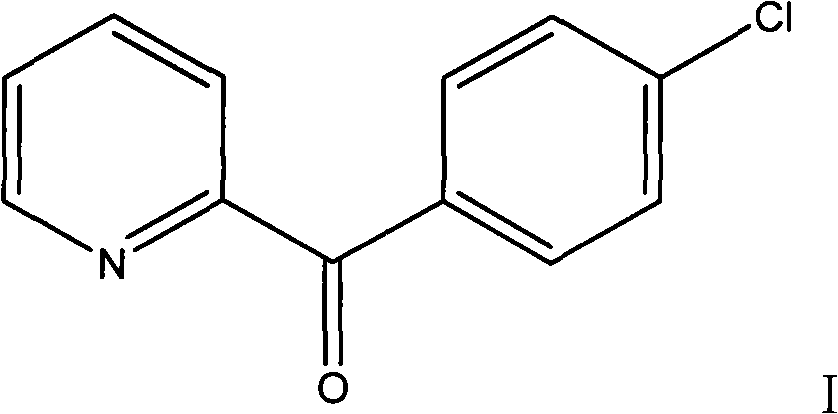
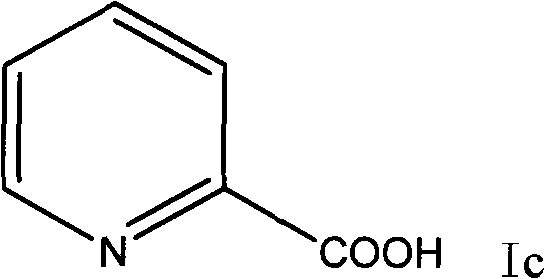
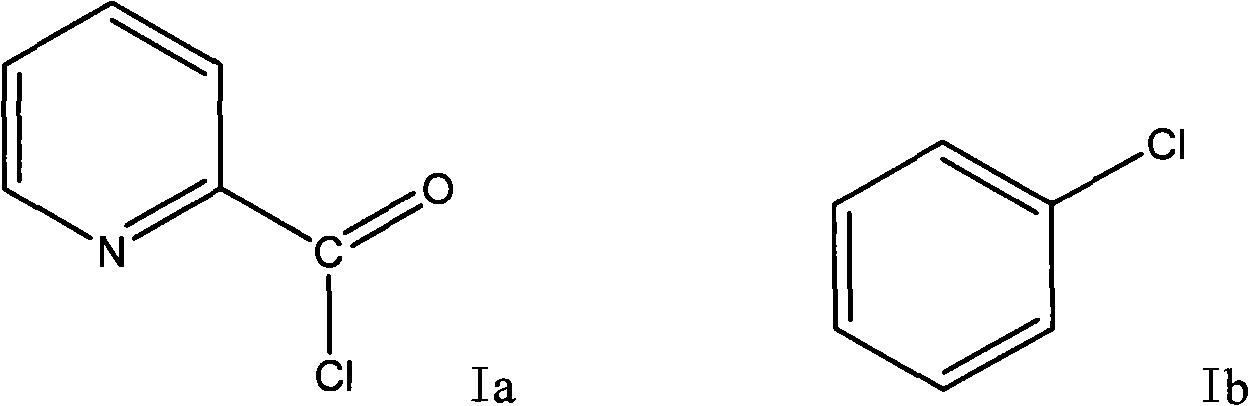Preparation method of 2-p-chlorobenzyl pyridine
A technology of chlorobenzylpyridine and chlorophenyl, which is applied in the field of preparation of intermediate 2-p-chlorobenzylpyridine, can solve the problems of many steps and high requirements for reaction conditions, and achieve low cost, high product purity and easy industrialization Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Preparation of 2-p-chlorobenzylpyridine
[0035] Add 2.46g of 2-pyridinecarboxylic acid into a 100ml reaction flask, then slowly add 10ml of thionyl chloride into the constant pressure dropping funnel, slowly raise the temperature to reflux, and react for 3 hours. Heating was stopped, and the remaining thionyl chloride was recovered under reduced pressure to obtain a dark red solid of 2-pyridinecarbonyl chloride hydrochloride. Add 20ml of chlorobenzene under the condition of ice bath, slowly add 5.34g of aluminum trichloride under stirring, continue to stir after adding, after half an hour, transfer the reaction bottle into an oil bath and slowly heat to about 100°C, and react for 3 hours , stop responding. Recover the remaining chlorobenzene under reduced pressure, and then slowly add ice cubes (made by adding 2ml of concentrated hydrochloric acid to 20ml of water) under ice bath conditions. The reaction is very violent, and ice cubes should be added careful...
Embodiment 2
[0037] Example 2 Preparation of 2-p-chlorobenzylpyridine
[0038] Add 2.46g of 2-pyridinecarboxylic acid into a 100ml reaction flask, then slowly add 10ml of thionyl chloride into the constant pressure dropping funnel, slowly raise the temperature to reflux, and react for 3 hours. Heating was stopped, and the remaining thionyl chloride was recovered under reduced pressure to obtain a dark red solid of 2-pyridinecarbonyl chloride hydrochloride. Add 20ml of chlorobenzene under the condition of ice bath, slowly add 7.24g of aluminum trichloride under stirring, continue to stir after the addition, after half an hour, transfer the reaction bottle to an oil bath and slowly heat to about 108°C, and react for 6 hours , stop responding. Recover the remaining chlorobenzene under reduced pressure, and then slowly add ice cubes (made by adding 2ml of concentrated hydrochloric acid to 20ml of water) under ice bath conditions. The reaction is very violent, and ice cubes should be added car...
Embodiment 3
[0040] Example 3 Preparation of 2-p-chlorobenzylpyridine
[0041]Add 2.46g of 2-pyridinecarboxylic acid into a 100ml reaction flask, then slowly add 10ml of thionyl chloride into the constant pressure dropping funnel, slowly raise the temperature to reflux, and react for 3 hours. Heating was stopped, and the remaining thionyl chloride was recovered under reduced pressure to obtain a dark red solid of 2-pyridinecarbonyl chloride hydrochloride. Add 20ml of chlorobenzene under the condition of ice bath, slowly add 6.7g of aluminum trichloride under stirring, continue to stir after adding, transfer the reaction bottle into an oil bath pot and slowly heat to about 145°C after half an hour, and react for 10 hours , stop responding. Recover the remaining chlorobenzene under reduced pressure, and then slowly add ice cubes (made by adding 2ml of concentrated hydrochloric acid to 20ml of water) under ice bath conditions. The reaction is very violent, and ice cubes should be added caref...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com