Donor-acceptor type based organic electroluminescent material
An electroluminescent material and donor-acceptor-type technology, which is applied in the fields of luminescent materials, organic chemistry, preparation of organic compounds, etc., can solve problems such as limiting molecular design, and achieve fewer synthesis steps, simple methods, and simple and easy purification methods. effective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Add malononitrile (1.87 g, 28.3 mmol) and isophorone (3.90 g, 28.3 mmol) to a mixed solution consisting of 28 μl acetic acid, 18 μl acetic anhydride, 380 μl piperidine and 8 ml N,N-dimethylformamide Stir at room temperature for 1 hour, then raise the temperature to 78°C and stir for 1 hour, then add 3.4526g of salicylaldehyde, stir at 78°C for 1 hour, pour the reactant into 200ml of 3% hydrochloric acid solution heated to 60°C while hot , stirred to produce a brick red precipitate.
[0039] The precipitate was collected, vacuum filtered, and the precipitate was washed with 100 ml of deionized water for 3 times. Recrystallize with a mixed solution of chloroform and ethyl acetate at a volume ratio of 3:1, use 50 ml of the mixed solution each time, and recrystallize 5 times to obtain purified 3-dinitrile methylene-5,5-dimethyl- 1-(2-Hydroxystyrene)cyclohexene (DCD(2-OH)SC), 65% yield.
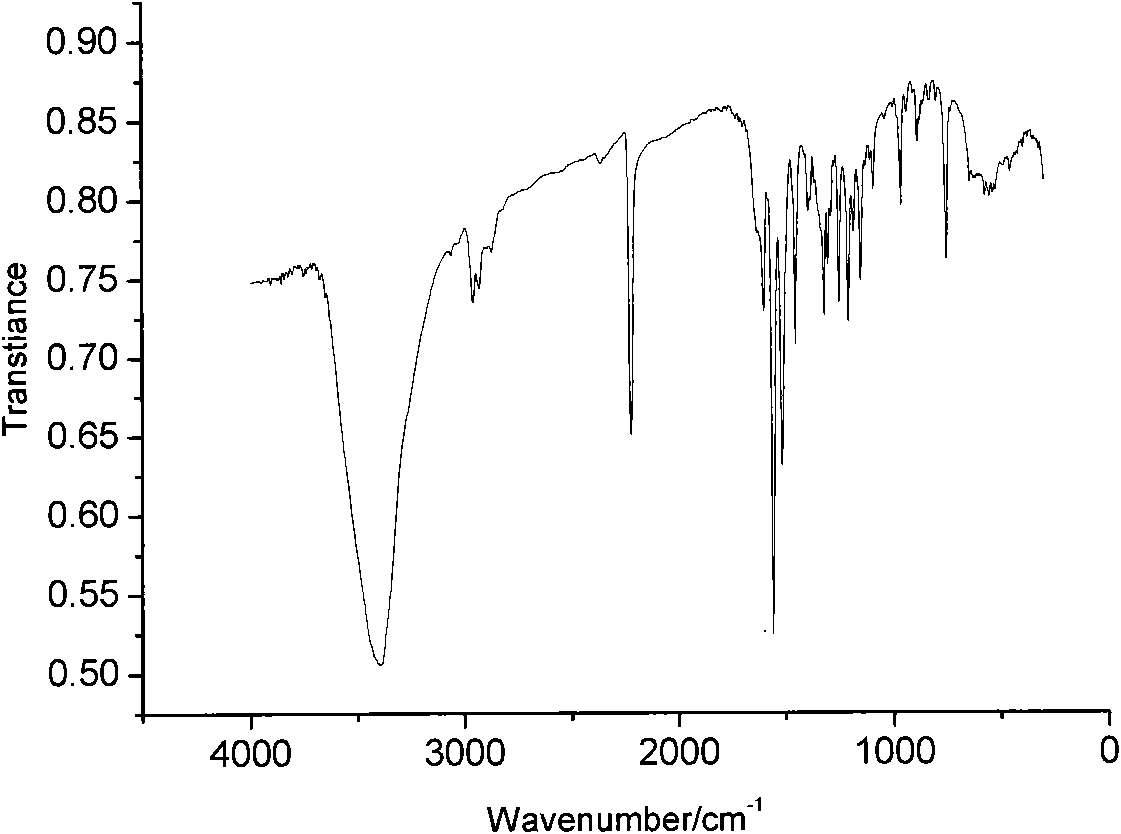
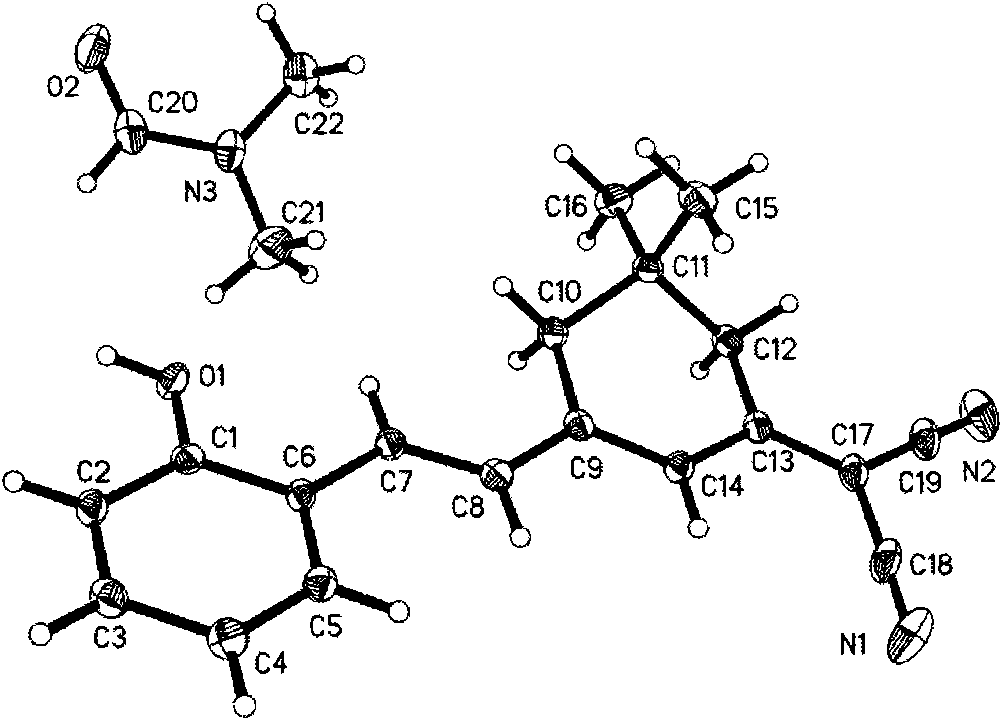
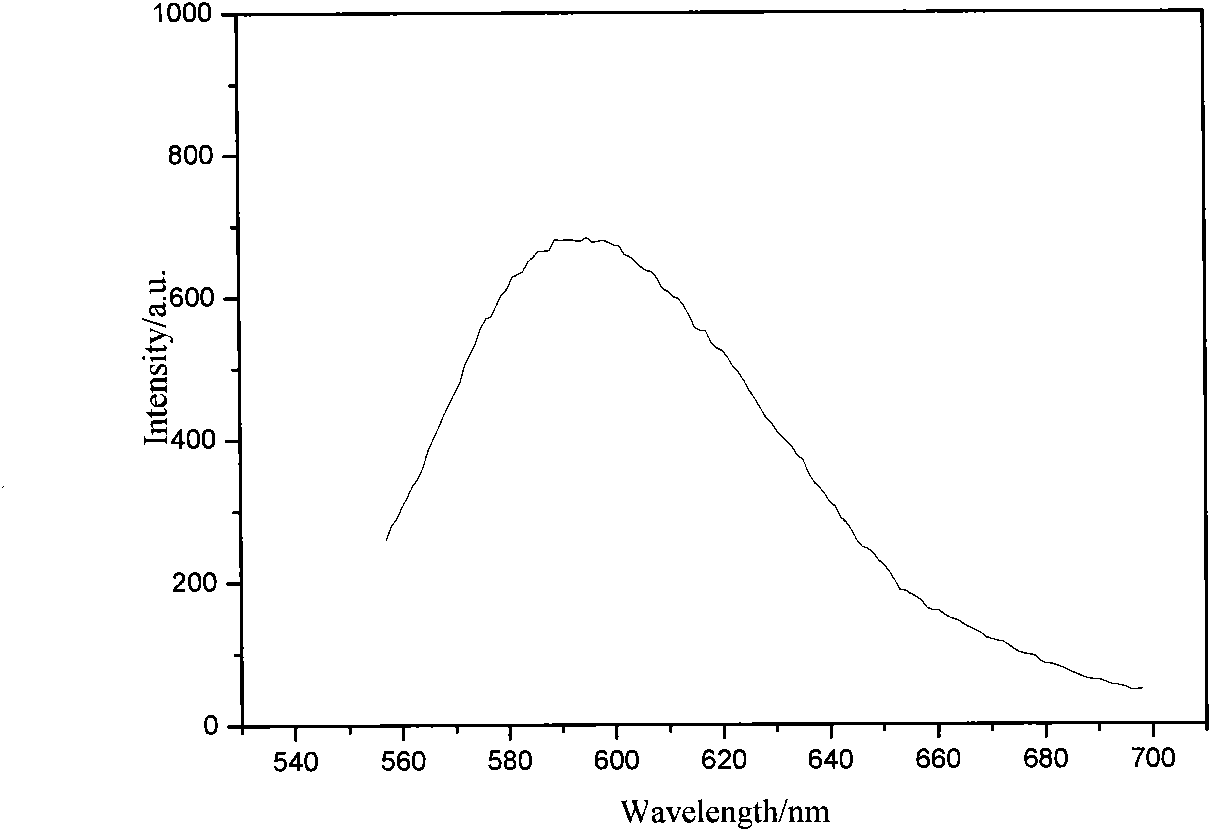
[0040] Characterize the chemical structure of the purified DCD(2-OH)SC:
[0041] 1. ...
Embodiment 2
[0055] Add 1.84 g of malononitrile and 4.05 g of isophorone to a mixed solution consisting of 30 μl of acetic acid, 19 μl of acetic anhydride, 407 μl of piperidine and 8.6 ml of N,N-dimethylformamide, stir at room temperature for 60 min, and then Raise the temperature to 80°C and stir for 1 hour, then add 3.74g of 3-hydroxybenzaldehyde, stir and react at 80°C for 1 hour, pour the reactant into 200ml of 3.25% hot hydrochloric acid solution heated to 60°C while it is still hot, and stir to produce brick red Precipitate.
[0056] Recrystallize according to the method of Example 1 to obtain brick red solid 3-dinitrile methylene-5,5-dimethyl-1-(3-hydroxystyrene) cyclohexene (DCD(3-OH)SC) Yield 84%.
[0057] 1. Elemental analysis:
[0058] The molecular formula of DCD(3-OH)SC is C 19 h 18 N 2 O, molecular weight 290.35, its elemental analysis (%) result is as follows:
[0059] Calculated: C, 78.52; H, 6.20; N, 9.64;
[0060] Measured values: C, 78.47; H, 6.17; N, 9.60.
[00...
Embodiment 3
[0066] Add 1.79 g of malononitrile and 3.80 g of isophorone to a mixed solution consisting of 26 μl of acetic acid, 17 μl of acetic anhydride, 359 μl of piperidine and 7.56 ml of N,N-dimethylformamide, stir at room temperature for 1 h, and then Raise the temperature to 75°C and stir for 1 hour, then add 3.4526g of 4-hydroxybenzaldehyde, stir and react at 80°C for 1 hour, pour the reactant into 200ml of 3.2% hot hydrochloric acid solution heated to 60°C while it is still hot, and stir to produce a brick-red precipitate thing.
[0067] Recrystallize according to the method of Example 1 to obtain brick red solid 3-dinitrile methylene-5,5-dimethyl-1-(4-hydroxystyrene) cyclohexene (DCD(4-OH)SC) , yield 93.4%.
[0068] 1. Elemental analysis:
[0069] The molecular formula of DCD(4-OH)SC is C 19 h 18 N 2 O, molecular weight 290.35, its elemental analysis (%) result is as follows:
[0070] Calculated: C, 78.52; H, 6.20; N, 9.64;
[0071] Measured values: C, 78.40; H, 6.17; N, 9...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface roughness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com