Organic conjugated polymer fluorescent material and its synthesis method
A technology of polyphenylene and phenylboronic acid, which is applied in the direction of luminescent materials, chemical instruments and methods, can solve the problems of increasing the difficulty and cost of material synthesis, unfavorable practical application, etc., and achieves low price, regular structure, and adjustable luminous range Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0094] In the present invention, the preparation method of polyparaphenylene and its derivatives, the polymer shown in general formula IA, IC or ID at least includes the following steps:
[0095] A method for preparing an organic conjugated polymer fluorescent material, the method comprising:
[0096] Step 1: Polymerize one item selected from items i-vii below under Suzuki coupling reaction conditions in an aqueous mixed solvent:
[0097] i. a precursor material shown in general formula A; ii. the precursor material shown in general formula A and halide or containing R 21 or R 22 The combination of the halide of the substituent; iii. The precursor material shown in general formula A and aryl borate or containing R 21 or R 22 The combination of the aryl borate compound of the substituent; iv. the combination of the precursor material shown in the general formula A and the precursor material shown in the general formula B; v. the precursor material shown in the general formul...
Embodiment 1
[0142] Example 1, mild aqueous phase synthesis of polyparaphenylene and its fluorescence characterization:
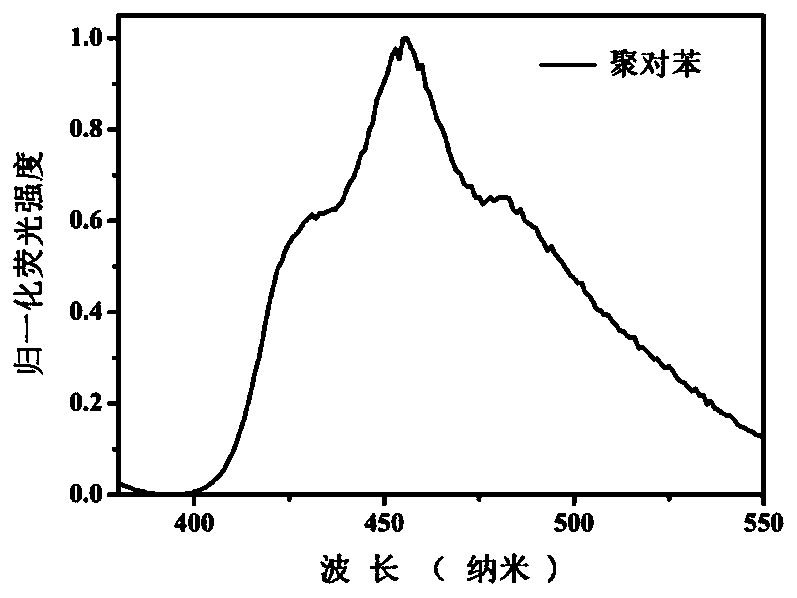
[0143] Example 1 provides a class of synthesis conditions for poly-p-phenylene. Under this condition, the fluorescence emission spectrum of poly-p-phenylene is in the blue light region, and the maximum emission wavelength is at 455 nm.
[0144] 4-Bromophenylboronic acid (0.2mmol, 41mg), sodium hydroxide (0.3mmol, 12mg), ethylene glycol (3ml), water (12ml) were added to a 20ml reaction flask and the reaction mixture was heated at 60°C and stirred for 6h, Add palladium acetate catalyst and 4-bromophenylboronic acid in a molar ratio of 1:100, heat the reaction mixture at 60°C and stir for 6 hours, wash off unreacted inorganic bases and monomers in the product with water, ethanol, and dichloromethane, and filter Obtain polyparaphenylene.
[0145] Test the fluorescence emission spectrum of poly-p-phenylene in ethanol solvent, the results are as follows: figure 1 shown. Th...
Embodiment 2
[0146] Embodiment 2 Structural characterization of polyparaphenylene synthesized in mild water phase:
[0147] Example 2 provides the structural characterization of the poly-p-phenylene synthesized under this condition, indicating that the poly-p-phenylene synthesized under this condition has a regular chain structure and a better conjugated structure, and has excellent thermal stability
[0148] Characterize the structure of polyparaphenylene:
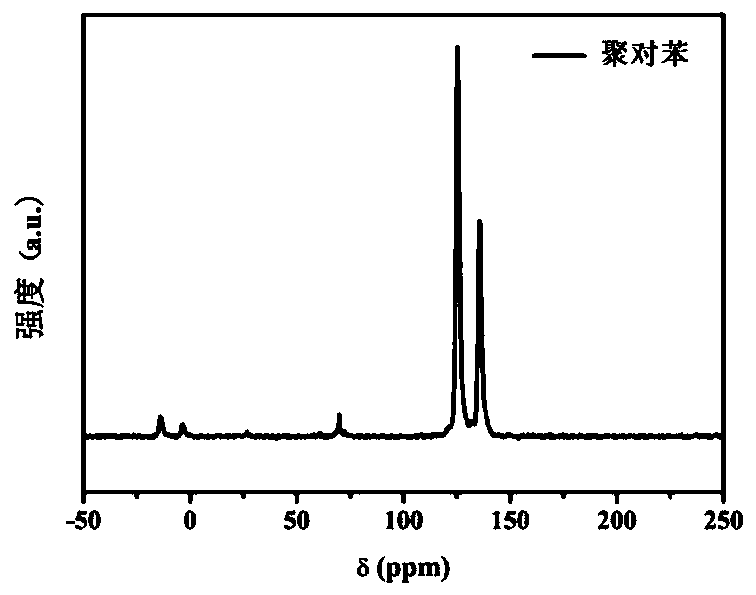
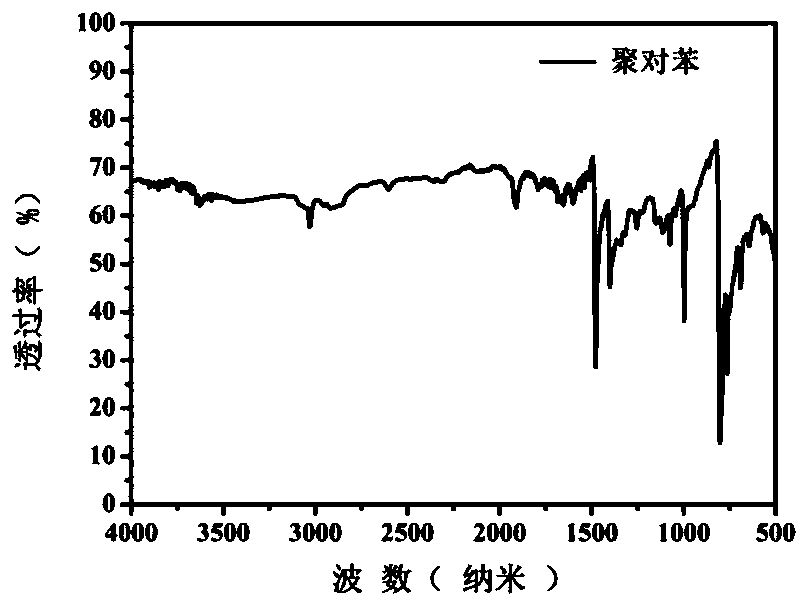
[0149] Solid-state carbon NMR spectrum, the results are as follows Figure 2a ), the peaks of poly-p-phenylene at 125.4ppm and 135.8ppm respectively correspond to the tertiary and quaternary carbon atoms on the para-substituted benzene ring; -1 The absorption peak is the para-position substitution on the benzene ring from poly-p-phenylene; X-ray powder diffraction spectrum, the result is as follows Figure 2c ), 3 strong and sharp peaks in the range of 19-30 ° show that poly-p-phenylene has regular repeating units and has good cryst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com