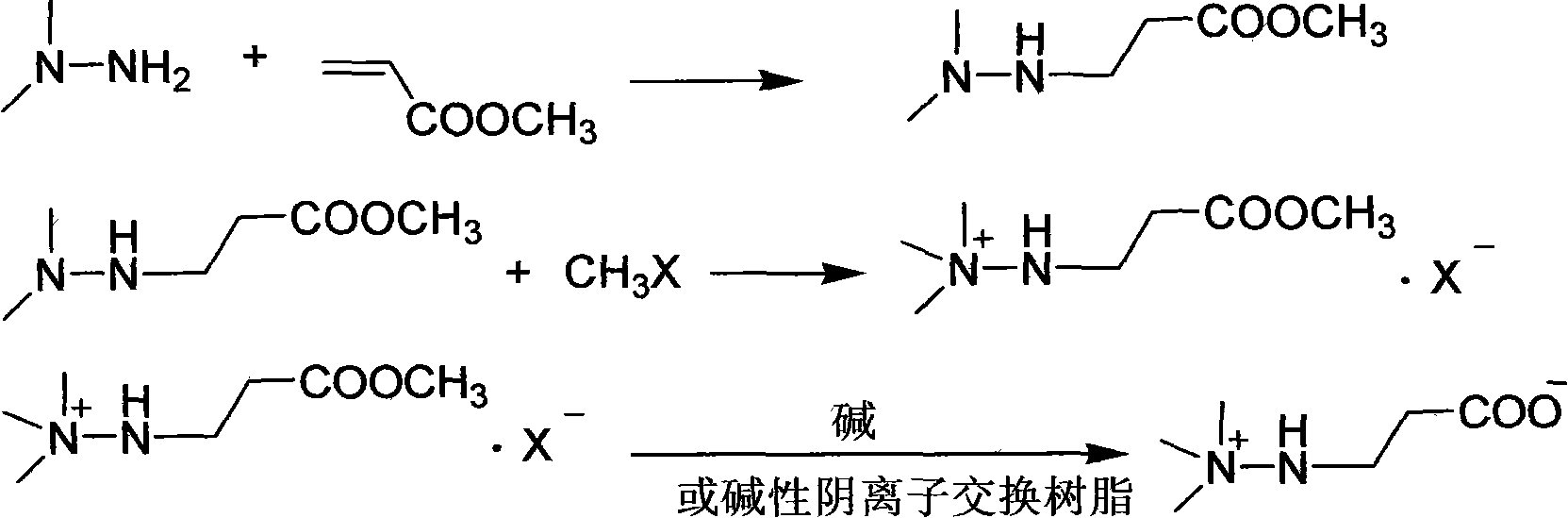
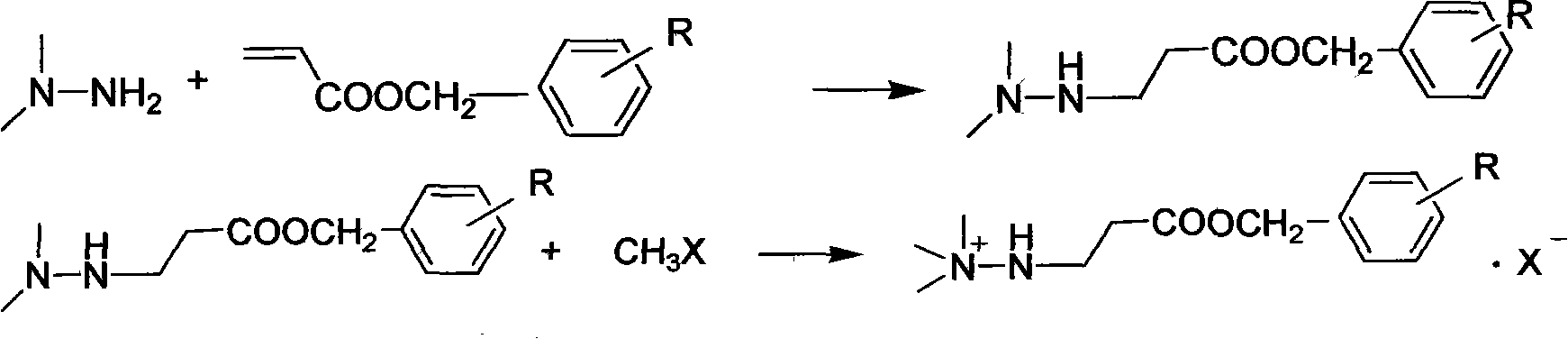
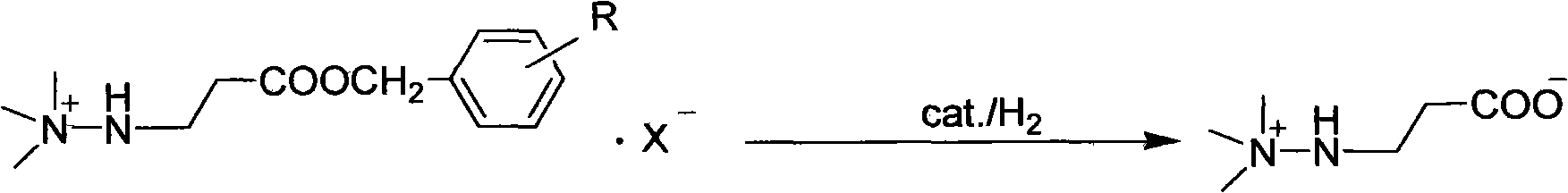
Preparation method of mildronate
A technology of meldonium and methylation, which is applied in the field of preparation of meldonium, can solve the problems of unsuitability for industrial production, difficulty in removal, difficulty in product quality meeting the requirements of raw materials, etc., and avoid inorganic bases and anion exchange resins. , the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 162g (1mol) of benzyl acrylate into a three-necked flask with mechanical stirring and dropping funnel, cool to 10°C, add 63g (1.05mol) of unsymmetrical dimethylhydrazine dropwise, and continue to stir at the same temperature after 2 hours. After 2 hours, 199.4 g of benzyl 3-(2,2-dimethylhydrazine)propionate was distilled under reduced pressure, with a yield of 89.8% and a content of 98.3%;
[0027] Dissolve the above distilled product in 200ml of ethanol, cool to 10°C, pass through 90.4g of methyl bromide within 1 hour, continue to stir at the same temperature for 1 hour, filter, wash with cold ethanol, and dry to obtain 3-(2,2,2-trimethyl hydrazine) benzyl propionate bromide 256.5g, yield 90.1%, content 98.5%;
[0028] Dissolve the above benzyl ester bromide in 150ml of water, add 5g of 5% Pd / C, heat to 50°C, feed hydrogen and keep the pressure at 0.3MPa, react for 2 hours, filter the catalyst, remove the solvent water under reduced pressure, and the residue Add 3...
Embodiment 2
[0030] The preparation of 3-(2,2,2-trimethylhydrazine) benzyl propionate bromide is the same as in Example 1;
[0031] Dissolve the above-mentioned benzyl ester bromide in 150ml of water, add 10g of Raney-Ni, heat to 60°C, pass in hydrogen and keep the pressure at 0.5MPa, react for 3 hours, filter the catalyst, remove the solvent water under reduced pressure, add ethyl acetate to the residue 300ml of ester, adjust the pH to 7-8 with triethylamine, stir for 10 minutes, filter, wash twice with ethyl acetate, and recrystallize the solid with 95% ethanol to obtain 106.6g of the target product, with a yield of 90.3% and a content of 99.6% .
Embodiment 3
[0033] The preparation of 3-(2,2-dimethylhydrazine) benzyl propionate is with embodiment 1;
[0034] Dissolve the above distillation product in 200ml of ethanol, cool to 10°C, add 134.5g of iodomethane dropwise for 1 hour, continue stirring at the same temperature for 1 hour, filter, wash with cold ethanol, and dry to obtain 3-(2,2,2- Trimethylhydrazine) benzyl propionate iodide 300.7g, yield 92%, content 99.1%;
[0035] Dissolve the above-mentioned benzyl ester iodide in 150ml of water, add 5g of 5% Pt / C, feed hydrogen at room temperature and keep the pressure at 0.2MPa, react for 2 hours, filter the catalyst, remove the solvent water under reduced pressure, and add dichloromethane to the residue 300ml, adjusted the pH to 7-8 with triethylamine, stirred for 10 minutes, filtered, washed twice with dichloromethane, and the solid was recrystallized with 95% ethanol to obtain 109.1g of the target product with a yield of 90.5% and a content of 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com