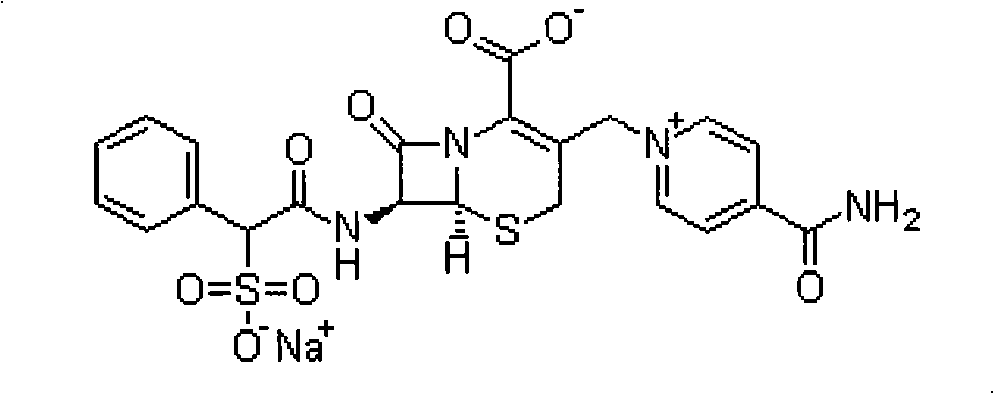
Process for purifying cefsulodin sodium by solvent crystallization method
A technology of cefsulodin sodium and sporoxalin, which is applied in the field of pharmacy, can solve the problems of high column chromatography cost, cumbersome operation, and low feasibility, and achieve the effects of stable product quality, simple operation, and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Dissolve 15.8g of sodium bicarbonate in 800ml of water, add 100g of cefsulodin at 0-5°C, the pH of the solution is 4.2-4.8, stir to dissolve, add 2.0g of activated carbon, decolorize for 0.5h, and filter. Control the temperature at 0-5°C, add 2500ml of ethanol and a small amount of seed crystals, and grow the crystals for 1 hour. Add 4000 ml of ethanol dropwise within 2 hours, grow the crystal for 1 hour, filter, and dry the filter cake in vacuum at 35°C for 3 hours to obtain 99.5 g of cefsulodin sodium with a yield of 95.4%, HPLC content of 99.7%, and related substances of 0.36%. (Conditions for HPLC determination of content: chromatographic column: Sephadex G-10 post, column temperature: 25°C, mobile phase: 0.05mol / L phosphate buffer (pH7.0), flow rate: 0.5ml / min , detection wavelength: 254nm, injection volume: 20μl, the same below)
Embodiment 2
[0055] Dissolve 10.0g of sodium carbonate in 600ml of water, control the temperature at 0-5°C, add 100g of cefsulodymic acid, the pH of the solution is 4.3-4.6, stir to dissolve, add 2.0g of activated carbon, decolorize for 0.5h, and filter. Control the temperature at 0-5°C, add 2,200ml of ethanol and a small amount of seed crystals, grow the crystals for 1 hour, add 3,800ml of ethanol dropwise within 2 hours, grow the crystals for 1 hour, filter, and dry the filter cake in vacuum at 35°C for 3 hours to obtain 100.4g of cefsulodin sodium. The yield is 96.3%, the HPLC content is 99.9%, and the related substances are 0.33%.
Embodiment 3
[0057] Dissolve 7.5g of sodium hydroxide in 600ml of water, control the temperature at 0-5°C, add 100g of cefsulodin, the pH of the solution is 4.2-4.5, stir to dissolve, add 2.0g of activated carbon, decolorize for 0.5h, and filter. Control the temperature of the filtrate at 0-5°C, add 2200ml of ethanol and a small amount of seed crystals, and grow the crystals for 1 hour. Add 3800ml of ethanol dropwise within 2 hours, grow crystals for 1 hour, filter, and dry the filter cake in vacuum at 35°C for 3 hours to obtain 99.8 g of cefsulodin sodium with a yield of 95.7%, HPLC content of 99.6%, and related substances of 0.32%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com